The term amniotic band syndrome is applied to a broad spectrum of sporadic congenital anomalies that involve the limbs, craniofacial regions, and trunk, ranging from constrictive bands, pseudosyndactyly to amputation, as well as multiple craniofacial, visceral, and body wall defects (1-8). The term amniotic band syndrome also encompasses amniotic band disruption complex (3), amniochorionic mesoblastic fibrous strings (1), aberrant tissue bands (2), amniotic deformity, adhesion and mutilation (ADAM) complex (9,10), amniotic adhesion malformation syndrome (11), and the limb body wall complex (12). It has been suggested that the term syndrome is inappropriate and a more correct term for amniotic band-associated anomalies is amniotic band sequence (ABS) as they are thought to occur as a consequence of amniotic bands.
Several theories have been advanced to explain the occurrence of these anomalies but two are most commonly held. In 1930, Streeter proposed that a disruption in embryogenesis at the time of the formation of the germ disk and the amniotic cavity initiated a chain of events leading to multiple defects (13). He suggested that amniotic bands were the result, not the cause, of the pathologic process. In 1992, Bamforth reviewed this theory in a series of 54 cases of ABS and concluded that it may be caused by a localized disturbance in the establishment of basic embryonic organization (12). The most widely accepted theory was proposed by Torpin in 1965 (1). He examined the placenta and fetal membranes in a number of affected individuals and concluded that the disorder was caused by primary rupture of the amnion early in gestation (3,4,9,11).
More recently, Moerman et al. (1992) proposed that the ABS is a collection of three distinct entities that can reconcile both Streeter’s and Torpin’s hypotheses (14). They suggested that ABS consists of three distinct lesions: (1) constrictive tissue bands; (2) amniotic adhesions; and (3) the more complex pattern of anomalies designated the limb–body wall complex (LBWC). In this report of the fetopathologic evaluation of 18 cases of ABS, 4 had clearly constrictive bands, which formed as a result of the amnion rupture sequence. The bands that resulted from amnion rupture encircled the limbs, resulting in annular constrictions, secondary syndactyly, and intrauterine amputations. In addition, constrictive bands involving the umbilical cord is a recognized cause of fetal death (1,15). These authors distinguish cases caused by constrictive bands from those caused by broad amniotic adhesions. Moerman et al. (1992) suggested that adhesive amniotic bands were morphologically and pathogenetically different from constrictive bands (14). Adhesive amniotic bands are usually associated with severe defects such as encephalocele and facial clefts. This group demonstrated pathologically that cranioplacental adhesions are broad adhesions, with the fetal skin fused to the amnion at the margins of the cranial defect. They speculated that the amnion covering the placenta or membranes seals the cranial defect separating the protruding brain from the chorion. Van Allen et al (1987)., proposed that the amnion becomes adherent to the embryo in areas of ischemic necrosis following vascular disruption. In short, the amniotic adhesions occur secondary to fetal defects (15).
Moerman et al. (1992) considered the LBWC to be due to both band-related and non-band-related defects. The band-related defects include limb defects such as club foot. Non-band-related defects occur as a result of vascular disruptions or from compression (16). The thoracoabdominoschisis of LBWC is characterized by an anterolateral body wall defect with evisceration of abdominal and/or thoracic organs. The eviscerated organs are in an extra-amniotic sac bounded by the chorionic plate, a persistent extraembryonic coelom. The amnion is continuous with the skin. The umbilical cord is extremely short, with umbilical vessels running in the amniotic sac, often with an absent umbilical artery. The severe scoliosis is a postural deformity caused by abnormal fixation of the fetus to the placenta. They also cite the high incidence of internal structural defects such as cardiac anomalies, unilateral absence of a kidney, or intestinal atresia, which do not fit with simple amnion rupture.
The fetal malformations that can occur as a result of ABS can be categorized into neural tubelike defects, craniofacial anomalies, limb anomalies, and constrictive bands (4,7,17-19). The neural tube-like defects include cases of anencephaly and encephalocele, which may be asymmetric or multiple. The craniofacial anomalies include facial clefts, nasal deformity, asymmetric microphthalmia, and abnormal cranial calcification. Limb anomalies may be multiple and asymmetric, including limb or digital amputation, pseudosyndactyly, abnormal dermatoglyphics, and clubbed feet. Abdominal wall and thoracic wall defects can occur, and some cases may be mistaken for gastroschisis or omphalocele with rupture.
The most puzzling component of the ABS is its association with visceral anomalies, including bladder exstrophy, vertebral hypoplasia, and other renal, gonadal, cardiac, and pulmonary defects (12). Constrictive bands involving the extremities are the most common defects observed in ABS (20).
It has been suggested that the variation in manifestations of the ABS are due to differences in timing of amniotic rupture and the degree to which the fetus becomes entangled by strands of amnion (3,4). The effects the amniotic bands have on the developing fetus have been classified into malformation, disruption, and deformation (3). Amniotic bands that interrupt the normal sequence of embryologic development lead to malformations such as cleft lip and palate, and abdominal wall defects. In contrast, bands may tear normally developed structures, leading to disruption such as central nervous system or calvarial defects, acrosyndactyly, amputations, and nonanatomical facial clefts (6). The effects of fetal compression and tethering may lead to deformations such as clubbing of the feet and angulation of the spine.
In most cases there is no clear cause for amnion rupture, but both maternal and fetal disorders have been reported in association with ABS, including epidermolysis bullosa and connective tissue diseases (21-23). More commonly, iatrogenic amnion rupture has been reported in ABS in association with invasive procedures including amniocentesis, fetoscopy, and thoracoamniotic shunt placement and has been referred to in these instances as “pseudoamniotic band syndrome” (24-28). Not all cases included in ABS are associated with amnion disruption but have internal visceral anomalies not directly attributable to amniotic bands or have disruptive defects with an intact amnion (28-29). The later cases have been purported to be due to vascular disruptive sequence.
The timing of amnion rupture has been suggested to occur between 28 days after conception to 18 weeks of gestation. If amnion rupture occurs prior to 45 days of gestation, the results are likely to be devastating, including severe skull defects and major visceral defects (20). Rupture occurring after 45 days of gestation is likely to result in more limited defects.
The cause of amnion rupture and band formation is not well understood, but it has been observed following amniocentesis (30). Late gestation bands, even in the absence of an amniocentesis, can also occur. Lage et al., reported ABS presenting at birth with multiple abnormalities of the extremities despite a normal sonographic appearance at 21 weeks of gestation (31). There have also been cases of ABS associated with underlying disease. Young et al. reported two cases in fetuses with Ehlers–Danlos syndrome type IV and one with osteogenesis imperfecta (22). They speculated that the premature amnion rupture may have been due to reduced or abnormal collagen in the amnion. There have been rare familial cases of ABS, and some teratogens, such as lysergic acid diethylamide and methadone, have been reported in association with the syndrome (1,17,29,33-35). Other significant exposures include misoprostol and maternal fever (14,36).
Chorioamniotic separation, occurring spontaneously or as a consequence of invasive procedures, is a potential cause of the ABS. The incidence of chorioamniotic separation diagnosed by ultrasound is reported to range from 1 in 187 to 1 in 4333 births (1,35-38). The natural history of chorioamniotic separation occurring in normal pregnancies was initially thought to be benign. However, Graf et al., reported a case of chorioamniotic separation that resulted in the formation of amniotic bands involving the umbilical cord, resulting in fetal death (39). The incidence of chorioamniotic separation may be even higher in cases of fetal surgery. In the same report, Graf and colleagues described 5 cases of chorioamniotic separation occurring in a series of 40 patients undergoing open fetal surgery. Three of the five fetuses had amniotic bands involving the umbilical cord, leading to fetal death in one. This report speculated that because the amnion is adherent and fixed to the umbilical cord, once formed, amniotic bands may retract to the cord, causing strangulation. Heifetz et al., in a review of ABS, reported that as many as 10% of cases had umbilical cord strangulation (40).
ABS is often misdiagnosed, especially in cases of early amniotic rupture. Infants affected by early amniotic rupture present with anencephaly, encephalocele, abdominal or thoracic wall defects, and severe limb abnormalities. The severity of the anomalies may obscure the cause, especially if the amniotic bands are not evident at birth. It has been estimated that a correct neonatal diagnosis of ABS is made in only 24% to 50% of patients without specialized genetic consultation (4). Because of the imprecision of diagnostic criteria and difficulties in accurately diagnosing ABS, the estimates of its incidence vary widely. The reported incidence ranges from 1 in 1200 to 1 in 15,000 livebirths (4,5,18,33). More recent estimates place the incidence of ABS at 1 in 1200 because of the more frequent recognition of an amniogenic cause for congenital anomalies (4,14,38). In a retrospective analysis of 3173 autopsies performed during a 14-year period, Czichos et al., found 744 cases of malformations of which 14 had anomalies thought to be a consequence of amnion rupture (41). This series, yielding an incidence of 1 in 226 among fetuses and newborns undergoing autopsy, suggests that it may be a more common cause of anomalies than generally appreciated. Hands are more frequently affected than feet, with the largest fingers (third, fourth, and second) more commonly afflicted (40).
ABS is associated with numerous antenatal sonographic features, as there are numerous forms of the syndrome and these features may occur as isolated problems or in combination. The earliest that amniotic bands have been seen is at 12 weeks of gestation, by endovaginal probe. The bands can be extremely difficult to detect sonographically and ABS is more often diagnosed by the effect that they have on fetal anatomy.
The effect of amniotic bands on the extremities may be manifested by absent digits or portions of limbs, or a swollen distal arm or leg resulting from constrictive amniotic bands (42).
ABS may affect the face with cleft lip or palate, asymmetric microphthalmia, or severe nasal deformity. Encephalocele may be a manifestation of ABS, especially when off-midline eccentrically placed.
Abdominal wall defects can be the result of ABS, typically with large defects with free-floating intestine herniated outside the abdomen. The characteristic appearance of an aberrant sheet or band of amnion attached to the fetus with resultant deformity and restriction of motion allows a diagnosis of ABS to be made (Figure 3). However, prenatal diagnosis is the exception rather than the rule.
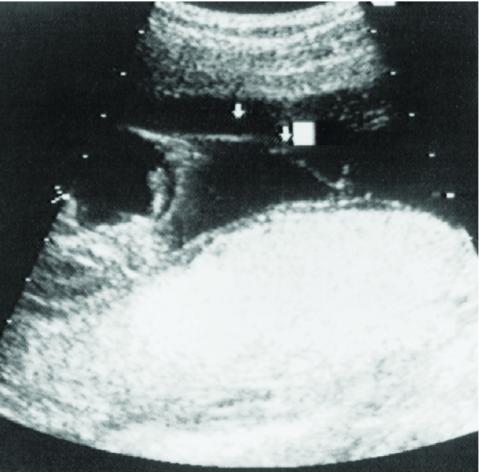
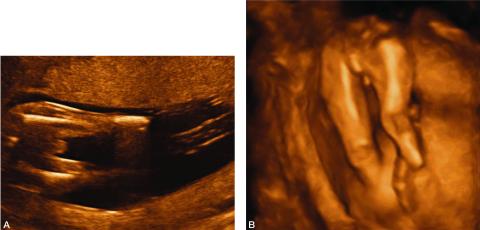
Figure 1: Sonographic image demonstrating an amniotic band attached to the fetus and floating in the amniotic fluid.
Figure 2. A. 2-D image depicting amputation of the distal portion of both lower extremities as a result of amniotic bands.
B. 3-D image of the same fetus showing bilateral limb reduction defects.
The findings in ABS may be limited to isolated defects, including isolated facial cleft, digital amputation, or mild elephantiasis of an extremity beyond a constrictive band (43,44). These isolated features may be difficult to diagnose sonographically because the detailed fetal visualization required is beyond the scope of routine obstetrical ultrasound examinations. At the worst end of the spectrum, the fetus may be so severely deformed by the amniotic bands that the spine is contracted and organs are formed in perplexing and bizarre proportions. The head may be completely misshapen or absent. The bands responsible for these deformities are rarely seen and a presumptive diagnosis of ABS is made based on the commonly associated deformities.
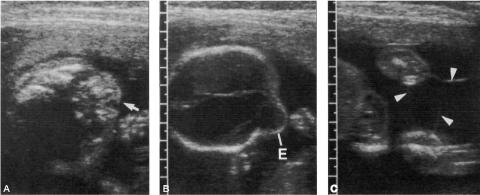
Figure 3: Sonographic image of a fetus with amniotic band syndrome manifesting as:
A. a ‘‘slash” defect in the maxillary region, and
B. an eccentric encephalocele.
C. Amniotic bands were also noted to be attached to the extremities (arrowheads).
The spinal deformities in ABS can be severe, manifesting as kyphotic lordosis or scoliosis as well as severe rotational abnormalities and even spinal amputation (45). While spinal deformity can be seen in other syndromes, severe spinal deformity should suggest ABS.
Spinal deformity associated with an abdominal wall defect is particularly suggestive of ABS. While the typical appearance of an omphalocele is possible, the more common defect is a large slashlike defect of both the thoracic and abdominal cavities with evisceration. These defects are associated with exteriorized bowel, liver, and sometimes heart without an enveloping membrane. When associated with limb abnormalities, this is characteristic of the LBWC form of ABS.
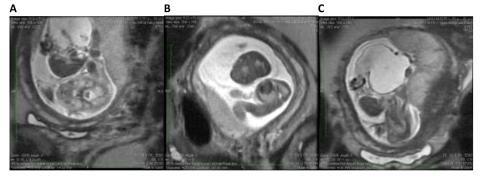
Figure 4. Fetal MRI of a fetus with limb–body wall complex showing severe twisting deformity of the spine associated with abdominoschisis and amniotic bands attached to the extremities.
A. The fetal MRI image demonstrates the contorted position of the fetus which in the same plane shows the axial view of the head and the adjacent completely extracorporeal liver and small bowel.
B. The fetal MRI image shows the fetal chest in axial plane demonstrating the very tiny chest and hypoplastic lungs.
C. The image shows the very short two vessel cord going from the large cystic structure which replaced the abdominal cavity directly into the placenta. These findings are most consistent with limb body wall complex.
Deformation of the calvarium is another group of anomalies characteristic of ABS. If complete, the fetus may appear anencephalic or to have acrania (46). If partial, the fetus may appear to have an encephalocele. The distinguishing features that characterize these defects as ABS are their asymmetric nature and associated spinal deformity or abdominal wall defects. In classic anencephaly, the calvarial bones are symmetrically absent. In anencephaly caused by ABS, there is some portion of calvarium present, usually near the base of the skull or near one or the other orbit. Similarly, classic encephaloceles occur near the midline, while ABS causes encephaloceles off the midline.
The presence of bands is unnecessary for the diagnosis of ABS in the presence of characteristic fetal anomalies. The sonographic detection of bands is helpful in confirming the diagnosis of ABS as the cause of fetal deformity. However, observation of these bands without fetal abnormality is not ABS. It is important for the sonographer to distinguish amniotic bands from other membranes and separations within the amnion. Separation of amnion and chorion is normal in early pregnancy until fusion occurs at approximately 16 weeks of gestation (47-49).
Chorioamniotic separation may occur as a result of amniocentesis or fetal surgery, and extrachorionic hemorrhage may separate the chorioamniotic membrane from the uterine wall (39,48,50). In both of these instances, a membrane may be observed sonographically. Other causes of membranes in the developing fetus include septate uterus, blighted twin, and circumvallate placenta (51).
Adhesions that form in the uterus as a result of curettage, cesarean section, or myomectomy may cause sheets of amnion that protrude into the lumen of the amniotic cavity (51-55). Randal et al., found that 76% of patients with amniotic sheets had undergone prior instrumentation (55). This results in an adhesion that becomes covered by chorion and amnion and has a thickness similar to the intertwin membrane of dichorionic diamniotic twins. These amniotic sheets do not adhere to the fetus because the amnion is intact (56). The uterine adhesion may rupture with growth of the fetus. Filly and Golbus have described the sonographic appearance of these synechiae as having a thickened base and a fine edge that undulates (51). There may be a bulbous edge, presumably due to the synechiae. There are no associated fetal abnormalities, and there is free fetal movement around the sheet. The synechiae may not be seen in the third trimester, because of rupture or compression by the growing fetus.
In the LBWC, there is a constellation of abnormalities, including myelomeningocele or caudal regression, thoracoabdominoschisis, or abdominoschisis and limb defects.
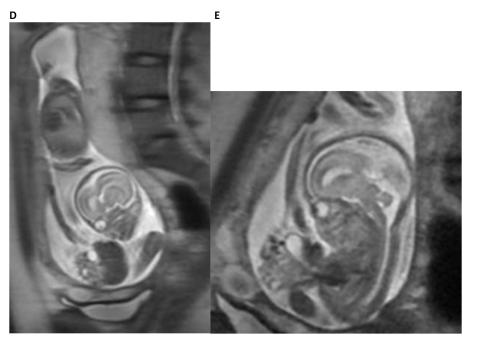
Figure 4. Fetal MRI of a fetus with limb–body wall complex showing severe twisting deformity of the spine associated with abdominoschisis and amniotic bands attached to the extremities.
D. The fetal MRI image demonstrates the contorted position of the fetus with the sagittal view of the head and the immediately adjacent completely extracorporeal liver, stomach and small bowel. In addition, the foreshortened two vessel umbilical cord is also seen in its entirety.
E. The fetal MRI image shows the fetal abdomen and chest in axial plane demonstrating the very tiny chest and herniated liver, stomach, small and large bowel with foreshortened umbilical cord. These findings are most consistent with limb body wall complex.
At least two of the three abnormalities listed above are necessary to make a diagnosis of LBWC. In LBWC, the umbilical cord is usually short but present. In body stalk anomaly, a similar constellation of findings may be seen but the umbilical cord is absent with the placenta attached directly to the fetus. If present in LBWC, there may be only a two-vessel cord. The limbs may be missing or the feet clubbed. The spine is often short and curved and sacral regression is common. There may be Arnold–Chiari malformation and hydrocephalus associated with the meningomyelocele. There may be ectopia cordis as part of the thoracoabdominoschisis. Facial clefts may also be seen in LBWC.
ABS involving the umbilical cord can be recognized by abnormal clustering of loops of umbilical cord entangled by a band, which may also be adherent to a limb. The cluster of umbilical cord loops will move together with movement of the involved limb. These findings may be subtle and should be sought in any case of ABS as umbilical cord involvement may result in fetal demise.
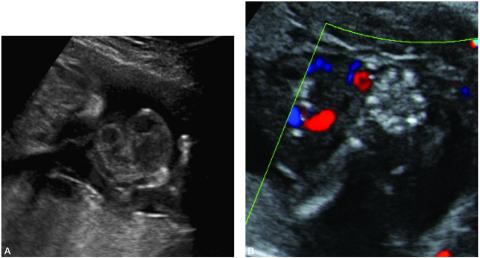
Figure 5. A. Sonographic image of amniotic bands involving the umbilical cord and the right hand and upper extremity. Movement of the hand would cause a cluster of umbilical cord loops to move together indicating amniotic band involvement of the umbilical cord.
B. Color Doppler of the same patient.
Extremity ABS should be assessed by pulse-wave and color Doppler to determine the severity of vascular compromise. Some constrictive bands may reduce the pulsatile blood flow distal to the band suggesting threatened limb loss.
Magnetic resonance imaging is an important adjunctive imaging technique which may provide more detailed information about the abnormalities which may help confirm the diagnosis or suggest alternative diagnoses (57,58). The wide field of view provided by MRI also provides assistance in planning the operative approach to fetoscopic lysis of amniotic bands.
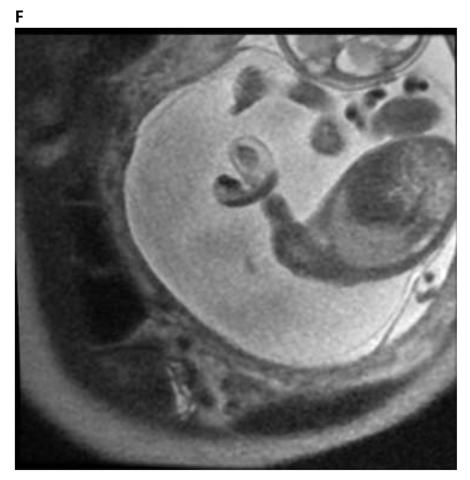
Figure 4. F. The fetal MRI image shows the effects of a constrictive amniotic band on the left upper extremities at the distal upper arm with distal edema. The Image also shows the umbilical cord in close proximity to the area of constriction suggesting umbilical cord involvement in amniotic band syndrome.
In cases of ABS involving encephalocele, fetal MRI plays an important role in evaluating the involvement of brain tissue, structural abnormalities and herniation in the encephalocele (58). Fetal MRI may also be helpful in assessing facial clefts in which may be difficult to determine the full extent of the clefting on ultrasound alone. Fetal MRI can also be used to measure fetal lung volumes in cases of abdominoschisis or thoracoshisis in which the lung development may be compromised causing severe pulmonary hypoplasia (60,61). This information may aid counseling of parents regarding prognosis and decisions around newborn resuscitation.
Fetal echocardiography is indicated in the evaluation of a fetus with suspected ABS when there is reason to suspect structural heart disease or if there is thoraco-abdominoschisis involving the heart or physiologic compromise of the heart. There is an increased incidence of congenital heart disease in cases of thoraco-abdominoschisis. The latter might occur if there was evidence of the formation of an arteriovenous fistula as result of a constrictive extremity band.
The differential diagnosis of ABS includes synechiae, uterine septa, residual gestational sac, fibrin strands, chorioamniotic separation, circumvallate placenta, syndromes and teratogens. Synechiae or sheets result from scar formation following cesarean section or uterine curettage and are extrinsic to the amniotic sac. The chorion and amnion fold over on themselves and look like a shelf rather than a band in cross-section (61,62). Synechiae do not attach to the fetus or restrict the movement of the baby. Synechiae may have blood flow detectable within them but amniotic bands are avascular. In contrast, a uterine septum originates in the fundus and is always sagittal in orientation. Septa are occasionally very thin and can be mistaken for an amniotic band.
A residual gestational sac from the demise of a co-twin will progressively decrease in size and disappear, unlike amniotic bands, which persist as the gestation proceeds. In the setting of in utero bleeding following an invasive procedure, fibrin strands may mimic amniotic bands. They may adhere to the fetus as amniotic bands do but they do not cause deformity and undergo lysis with time. Chorioamniotic membrane separation may follow any invasive procedure or can occur spontaneously (17,19,63,64). This usually has the appearance of a floppy membrane and may be crescentic in shape paralleling the chorionic plate and does not adhere to the fetus.
In cases of limb or digital amputation, amniotic bands are common but can also be due to limb reduction defects due to genetic or teratogenic causes. Transverse terminal limb deficiencies can be indistinguishable from ABS but are not associated with amniotic bands. The most common genetic syndrome associated with transvers limb deficiency is Adams-Oliver syndrome. This syndrome is characterized by asymmetric transverse terminal limb defects, aplasia cutis, and deficiency of the boney calvarium (65). Longitudinal limb deficiency due to sporadic or inherited mutations or poor first trimester glycemic control or teratogen exposure is associated with amniotic bands. Vascular disruptive sequence is a more common in monochorionic twins and has been reported following fetoscopic laser treatment of twin-twin transfusion syndrome (64-68).
The differential diagnosis in ABS depends on the sonographic findings. In isolated constrictive amniotic bands associated with distal limb edema, possible lymphatic or vascular malformations should also be considered. However, color Doppler studies should show the flow characteristics of a vascular malformation. Constrictive bands involving the upper extremity should suggest the possibility of the VACTERL association if the radius is affected, and Fanconi anemia if radial hypoplasia or absent thumbs are observed.
A diagnosis of LBWC requires two of three of the following abnormalities: (1) myelomeningocele or caudal regression, (2) abdominal or thoracoabdominal wall defect, or (3) limb defects. The main differential diagnoses are cases of isolated neural tube defects or ruptured omphalocele, which do not meet the criteria for LBWC. The body stalk anomaly has a similar constellation of anomalies but the placenta is attached to the trunk of the fetus.
There is great controversy about the pathogenesis of the various forms of ABS. Part of this controversy involves the timing in gestation of the development of amniotic bands. However, in constrictive amniotic bands of the extremities, the progression of constriction combined with fetal growth has resulted in extremity amputation (65-68).

Figure 2. A. 2-D image depicting amputation of the distal portion of both lower extremities as a result of amniotic bands.
B. 3-D image of the same fetus showing bilateral limb reduction defects.
ABS can be associated with either polyhydramnios or oligohydramnios. Despite the severity of some forms of ABS, there are no adverse maternal consequences for this diagnosis. The incidence of intrauterine fetal death from ABS involving the umbilical cord is not known but numerous cases have been reported (1,39,69). However, the poorly characterized pathogenesis of this syndrome and limited sonographic surveillance, limit our understanding of its prenatal natural history.
ABS is a relatively common, if underappreciated, cause of fetal and neonatal morbidity and mortality. The fetal lamb model of ABS has been useful to define the pathophysiology of ABS and to provide a tool to understand the unique fetal response to tissue injury, repair, and regeneration. Sonographic identification of ABS affecting the umbilical cord may be an indication for fetoscopic surgical intervention. Intervention for nonlethal limb deformation may also be considered if maternal risk is sufficiently low. ABS is another in a growing list of conditions for which fetal surgery may be considered.
Constrictive bands most commonly affect the extremities, but can also involve the umbilical cord, with resulting fetal death. Kanayama et al., described the reversal of diastolic flow observed in a fetus with umbilical cord constriction due to amniotic bands (69). Graf et al. (1997) similarly reported a case of amniotic bands involving the umbilical cord following the development of chorioamniotic separation (39). Despite initially normal umbilical artery Doppler waveforms, this fetus died within 2 weeks from a constrictive amniotic band of the umbilical cord. Reports have described constrictive amniotic bands as a cause of fetal death (1,14). However, until the reports by Kanayama and Graf and their colleagues, this was a diagnosis made pathologically after the fact. It is in cases like these, fetoscopic lysis of amniotic bands can be lifesaving (see Fetal Intervention below) (39,69). Cases of ABS, by definition, have disrupted membranes and typically deliver prematurely at 32-36 weeks.
In managing a pregnancy with suspected ABS, it is essential to have a detailed sonographic fetal survey to accurately assess any anomalies present. Fetal echocardiography is indicated in cases of abdominal wall or abdominothoracic wall defects because of the increased incidence of associated cardiac defects. Amniocentesis is not necessary in clear-cut cases of ABS, as these are sporadic deformations with no association with chromosomal abnormalities. However, in instances in which the diagnosis is uncertain, genetic amniocentesis should be considered. For example, in cases of abdominal wall defects in which a ruptured covered omphalocele cannot be excluded, genetic amniocentesis is indicated.
A fetus with ABS should pose no increased risk for the mother in the management of the pregnancy. There is no indication for cesarean section, except for obstetrical indications. In severe cases of ABS, such as LBWC, in which survival is not anticipated, early delivery by conventional labor and vaginal delivery without intervention for fetal distress should be considered.
The indications for fetal surgery are, with few exceptions, only for life-threatening conditions such as congenital pulmonary airway malformation (CPAM) with hydrops, diaphragmatic hernia with a low lung-to-heart ratio, bladder outlet obstruction with oligohydramnios, or sacrococcygeal teratoma with hydrops.
However, as experience with the techniques of fetal surgery has grown and the natural histories of certain non-life-threatening conditions have been better defined, the indications for fetal surgery have been extended. Two examples of this are in utero repair of meningomyelocele to prevent the devastating neurologic injury to the spinal cord (70) and fetoscopic cord ligation in monochorionic twins with imminent death of one twin to preserve the life and neurologic integrity of the surviving twin (71). The indications for fetal surgery in the ABS may be either for a life-threatening condition if it involves constriction of the umbilical cord or, more commonly, threatened limb amputation due to amniotic band constriction of the extremity (1,72-86).
Torpin reported 36 cases of fetal death due to cord constriction from amniotic bands. In each case, the diagnosis was made retrospectively (1). Recognition of amniotic bands constricting the umbilical cord has been reported by Kanayama et al., who were able to document fetal compromise by reversal of diastolic flow in the umbilical artery by color Doppler (69). It is in cases like the one reported by Kanayama et al. that fetoscopic lysis of amniotic bands can be lifesaving.
The rationale for performing fetoscopic lysis of constricting extremity amniotic bands is based on the prenatal natural history that progressive compromise of fetal growth leads to amputation. However, this assumes that the procedure can be accomplished with no maternal morbidity and minimal fetal morbidity. This procedure is hard to justify in the face of a serious maternal complication or a fetal death due to severely premature delivery at 21 or 23 weeks of gestation, even in the face of certain fetal limb amputation.
There is no standard approach to fetoscopic lysis of amniotic bands and reports have ranged from single port to two port techniques using laser or endoshears to cut the band, respectively. These are extremely challenging fetoscopic cases as the amniotic fluid volume is normal or low and there is extensive disruption of the amnion with bands visible throughout, though not necessarily involving the fetus, but potentially obscuring visualization. All cases of ABS have disruption of the amnion and the fetoscope compounds this by adding puncture of the chorion to gain access to the baby. Involvement of an extremity can be difficult to treat unless the fetus is paralyzed with intramuscular injection of rocuronium as any touch of the involved limb will cause withdrawal of the limb.
Even in cases in which the fetus is paralyzed, the amniotic band has usually cut through at least the skin and subcutaneous tissues, if not muscle and tendon. In fact, ABS can result in fracture of the bones of the involved limb. The amniotic band is often deeply embedded in the depth of the wound caused by the constrictive band. Endoshears or a laser fiber can be used to cut the amniotic band which should then be removed circumferentially from the depth of the wound. Similarly, release of umbilical cord amniotic bands can be equally challenging as the bands tend to wear away Wharton’s jelly and the umbilical vessels are exposed deep to the amniotic band. In cuttting the amniotic band, either with endoshears or laser fiber, there is the risk of lacerating the underlying umbilcal vessels. In fetoscopic release of a symptomatic ABS, inspection of all fetal extremities and the entire length of the umbilical cord is indicated to exclude the presence of asymptomatic bands that are almost always found and should be released before they become severe enough to cause symptoms.
On the basis of their experience with fetoscopy for cord ligation in TRAP sequence and the experimental work by Crombleholme et al. demonstrating the potential for functional recovery of banded extremities once released, Quintero et al. performed the first fetoscopic lysis of amniotic bands in human fetuses (72,73). Their first case was a fetus at 21 weeks of gestation with bilateral cleft lip and bands attached to the face and left upper extremity with distal limb edema. In order to avert limb amputation, fetoscopic lysis of bands was attempted at 22 weeks of gestation using a two-port technique. However, because of bleeding encountered on insertion of the second operating port, it was removed and the lysis was performed under ultrasound guidance. There was resolution of the distal edema within 6 days of the procedure.
At 32 weeks, microphthalmia and anophthalmia of the right orbit were first noted at the site of the previously attached amniotic band. The infant was delivered at 39 weeks and was found to have a type IV Tessier craniofacial cleft and right microphthalmia. The extremity showed minimal residual scarring where the band had been attached and lysed. The infant’s hand had radial paresis and mild hypoplasia. The second case was a fetus at 23 weeks of gestation with a thick amniotic band constricting the left ankle of the fetus. There was marked edema distal to the band and minimal blood flow to the foot was observed by color and pulsed Doppler. Fetoscopy was attempted but bleeding was encountered on insertion of the operating port, necessitating its removal. Attempts at ultrasound-guided lysis using endoscissors were unsuccessful. A 2.4-mm 0-degree operating scope with a 400-μm contact YAG laser fiber was used to lyse approximately 85% of the band. The edema markedly improved, as did distal arterial blood flow, and there was return of flexion and extension on follow-up sonographic examination. The infant delivered at 34.5 weeks and underwent Z-plasties for residual effects of the amniotic band.
The experience reported by Keswani et al., similarly supports the use of fetoscopic release of amniotic bands for limb salvage (76). However, the sequelae of the ABS may not completely resolve or may result in secondary lymphedema.
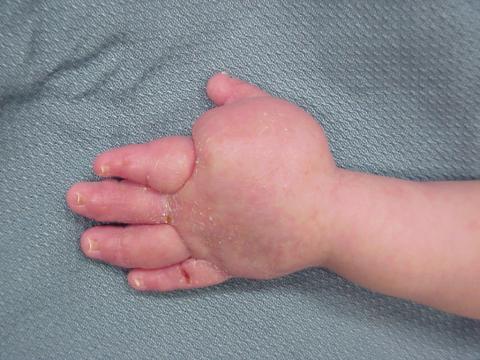
Figure 6. Photograph of the hand of a baby with secondary lymphedema who underwent fetoscopic release of amniotic band at the wrist causing critical ischemia. The hand was saved but there was significant lymphedema as a consequence of the tourniquet effect of the amniotic band.
It is worth noting that the cases reported all had additional amniotic bands encircling limbs not appreciated by ultrasound examination that were also lysed. Snyder et al. reported their experience with expectant management vs. fetoscopic release of amniotic bands in 24 patients with 5 involving the umbilical cord (81). There were no differences in treated versus untreated ABS with regard to maternal age, parity, rate, diabetes, prenatally diagnosed abnormalities, distal deformity, lymphedema, and amputation. The severity of ABS appeared to be worse in the fetoscopically treated ABS, however, based on the higher rate of abnormal vascular flow (5/10 versus 1/13) and cord involvement (7/10 versus 1/13). The indications for fetoscopic treatment of ABS was the presence of lymphedema or abnormal extremity blood flow distal to the amniotic band, or the presence of cord entanglement.
Figure 7. Movie of fetoscopic release of amniotic band causing critical ischemia of the fetal left hand which was relased using Diode laser in contact mode.
The indications for treatment of additional unsuspected amniotic bands noted at the time of fetoscopy was the presence of the band encircling an extremity with or without distal findings and any involvement of the umbilical cord. Contraindications to fetoscopic release included the band not involving the cord or the fetus, causing severe encephalocele, or extremity amniotic bands in twins in which the procedure would put the uninvolved twin at risk. In contrast, umbilical cord ABS in a monochorionic twin would still be an indication in that the life and neurologic integrity of the uninvolved twin would be at risk in the event of the death of the baby with ABS involving the cord.
The median gestational age at delivery in the series reported by Snyder for the operative group was 29 weeks (range 25 to 34) vs 36 weeks (range 25-39) for those managed expectantly. The survival in the operative group overall was 80%, but 86% in the cases in which there was umbilical cord involvement. This is the largest single center experience with ABS treated by fetoscopic release and first successful treatment of umbilical cord ABS. There have been numerous individual case reports and one other small series of cases of ABS treated fetoscopically with similar findings (73-86). All cases of ABS are at risk for prematurity due to the disrupted amnion. However, the gestational age at delivery of ABS treated by fetoscopic release was significantly less than observation alone. Excellent survival in ABS involving the umbilical cord however, can only be achieved with fetoscopic release in which the natural history is usually associated with intrauterine fetal demise.
While extremity ABS may have devastating morphologic and functional effects on a limb, possibly resulting in amputation, it is not lethal. Extremity ABS is not an indication for fetoscopic surgery unless maternal risks and incidence of preterm labor are fully appreciated by the mother. However, there are forms of ABS that are lethal or have devastating neurologic sequelae that may justify the current risks of intervention. Torpin has reported 36 cases of constrictive amniotic bands of the umbilical cord, which were uniformly fatal (1). Although rarer than other forms of ABS, umbilical cord involvement, once diagnosed sonographically, is amenable to fetoscopic release to avert fetal death (69,81).
LBWC in the setting of monochorionic twins is also an indication for fetal intervention to protect the normal co-twin, due to the high rate of intrauterine fetal demise. LBWC complex is uniformly fatal but up to 50% will succumb to intrauterine demise as opposed to neonatal death. In monochorionic twins, LBWC in one twin puts the normal co-twin at risk for demise and severe neurologic injury in the event of intrauterine demise. The options for intervention in twins complicated by LBWC include selective fetoscopic laser photocoagulation of the communicating vessels on the chorionic plate to protect the co-twin or selective reduction by means of intra-fetal radiofrequency ablation of the cord of the fetus with LBWC. Which option is best may depend upon whether parents would be comfortable with selective reduction versus separation of the vasculature on the chorionic plate.
The delivery plan depends on the nature of the ABS diagnosed. In cases of LBWC, a palliative care plan should be in place in consultation with Neonatology and the family to optimize the limited time the family will have with the baby and ensure that the baby is comfortable. In LBWC, there is a high rate of intrauterine fetal demise (up to 50% of cases), which the family should be aware of. This may be most appropriate at a facility close to the home of the parents, but in some cases, a local hospital may not be up to providing comfort care. In such cases, referral to a tertiary obstetrical unit may be indicated.
Most cases of ABS deliver early, usually in the range of 30-36 weeks, likely secondary to the extensive disruption of the amnion. This is true even if there has been no fetal intervention undertaken and adequate preparation of the family should be made to anticipate an early delivery in all ABS patients. In most instances of ABS, Cesarean section should be reserved for obstetric indications. In rare cases of ABS may result in fetal position or deformity which may cause dystocia which may warrant surgical delivery.
The site of delivery in ABS depends on whether or not a full resuscitative effort will be made for the baby. In cases of LBWC, comfort care will be the most appropriate approach in the delivery room and if possible, this should be planned at a hospital closest to home comfortable with caring for families in this difficult situation. In cases of ABS which affects the limbs, umbilical cord, or craniofacial regions in which a full resuscitation is anticipated, the baby should be delivered in a facility with
Neonatologists and appropriate pediatric surgical subspecialists are immediately available. Depending upon the nature of the ABS, this may include Pediatric Plastic and Reconstructive Surgeons, Pediatric Neurosurgeons, and Pediatric Orthopedic Surgeons.
Almost all cases of ABS can be delivered vaginally except when there are obstetrical indications for cesarean delivery. This may range from inability to tolerate labor to tethering which due to abnormal fetal position may cause dystocia. Due to the uniformly lethal nature of LBWC, cesarean delivery should be avoided, if at all possible, and the baby will be delivered with the placenta.
It is important to recognize that most cases of ABS have extensive disruption of the amniotic membrane, and PPROM is very common, with the majority of babies with ABS delivering between 30 and 36 weeks’ gestation.
Except in cases in which comfort care is planned for a baby with LBWC form of ABS, standard delivery room resuscitation should be anticipated for most babies with ABS.
In cases of ABS complicated by LBWC, extensive encephalocele, or thoraco-abdominoschisis, it is advisable to have a birth plan in place with the family to anticipate the needs of these babies and their families at the time of delivery. In many settings, a Perinatal Palliative care consultation may help prepare the family as well as the staff attending the delivery to facilitate making this difficult time as positive as possible for the mother and family.
Postnatal Evaluation
A fetus known to have ABS should be delivered in a tertiary care center with Neonatologists, Pediatric Plastic and Reconstructive surgeons, and Pediatric Orthopedic surgeons available. Treatment depends on the nature of the ABS and the severity of the deformation. In cases of umbilical cord involvement, early or even emergency delivery may be indicated if there are signs of fetal compromise (69). After delivery, a careful physical examination should assess the severity of the ABS. Often there will be no evidence of the amniotic band at the time of delivery. In the case of extremity amniotic bands, treatment is dictated by the severity of the deformation. The severity of deformity can range from a mildly constrictive band, requiring release, to near amputation, requiring debridement. More often, there is a bandlike deformation that requires Z-plasties to surgically correct it (87-89).
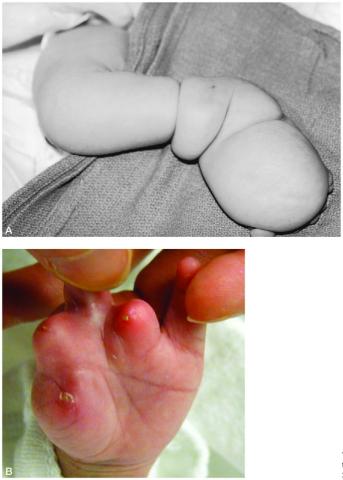
Figure 8. A. Postnatal appearance of the leg of a newborn with extremity amniotic band syndrome.
B. Postnatal appearance of the hand of a newborn with extremity amniotic band syndrome that resulted in digital amputation.
Postnatal Imaging
There is a limited role for postnatal imaging in ABS. However, in extremity ABS, plain radiographs should be obtained to rule out underlying boney fracture. If ABS results in encephalocele or facial clefts, ultrasound and MRI or CT scans should be obtained to assess impact of the bands on the central nervous system structures and craniofacial development with three dimensional reconstructions to assist in planning reconstructive procedures.
Medical Management
In cases of amniotic bands involving the face and head, there may be severe facial clefts, anophthalmia, and encephalocele. These deformities may require many extensive reconstructive procedures to achieve an acceptable cosmetic result. Cases of the LBWC form of ABS are always fatal, and no reconstructive procedures are indicated.
Outcomes
The outcome in ABS depends on the severity of the deformation. Cases of extremity ABS usually have an excellent long-term outcome. Even in cases of limb amputation, ambulation is possible with the aid of a prosthesis. The cosmetic results following extensive craniofacial reconstructive surgery are often acceptable, but the severity of these defects may leave these children permanently disfigured. ABS in which fetoscopic release was performed may show evidence of secondary lymphedema, which may require multiple surgical procedures to provide an acceptable functional result.
Most cases of ABS are sporadic and there is no risk of recurrence in subsequent pregnancies. There have been cases of ABS associated with underlying disease, such as Ehlers–Danlos syndrome type III or osteogenesis imperfecta. Similarly, ABS has been reported in association with teratogens such as methadone and lysergic acid diethylamide (33,34). While associated maternal disease or teratogenic exposure may predispose to recurrence, these are rare causes of the ABS. Some cases included in ABS are not easily explained by either amniotic band or vascular disruptive sequence. Some cases have been attributed to single gene mutations (4,14). Mutations in the human homologue of the mouse gene D “disorganization gene” have also been suggested as a cause of internal malformations observed in ABS (29,32,90). In addition, a combination of disruptive factors, amniotic bands, vascular disruption, and/or a mutation may be responsible in combination for some cases of ABS (36,91,92). Rare familial cases and concordance in monozygotic twins have been reported, supporting a genetic etiology in some instances (38,93,94).
1. Torpin R. Amniochorionic mesoblastic fibrous strings and amniotic bands. Am J Obstet Gynecol. 1965;91:65-75.
2. Jones KL, Smith DW, Hall BD, et al. A pattern of craniofacial and limb defects secondary to aberrant tissue bands. J Pediatr. 1974;84: 90-95.
3. Higginbottom MC, Jones KL. The amniotic band disruption complex: timing of amniotic rupture and variable spectra of consequent defects. J Pediatr. 1979;96:544-549.
4. Seeds JW, Cefalo RC, Herbert WNP. Amniotic band syndrome. Am J Obstet Gynecol. 1982;144:243-248.
5. Ray M, Hendrick SJ, Raimer SS, et al. ABS. Int J Dermatol. 1988;27:312-314.
6. Lockwood C, Ghidini A, Romero R, et al. ABS: reevaluation of its pathogenesis. Am J Obstet Gynecol. 1989;160:1030-1033.
7. Seidman JD, Abbondanzo SL, Watkin WG, et al. ABS: report of two cases and review of the literature. Arch Pathol Lab Med. 1989;113:891-897.
8. Kulkarni ML, Gopal PV. Amniotic band syndrome. Indian Pediatr. 1990;27:471-476.
9. Keller H, Neuhauser G, Durkin-Stamm MV, et al. “ADAM complex” (amniotic deformity, adhesions, mutilations): a pattern of craniofacial and limb defects. Am J Med Genet. 1978;2:81-98.
10. Orioli IM, Ribeiro MG, Castilla EE. Clinical and epidemiological studies of amniotic deformity, adhesion, and mutilation (ADAM) sequence in south American (ECLAMC) population. Am J Med Genet A. 2003; 1189:135-145.
11. Herva R, Karkinen-Jaaskelainen M. Amniotic adhesion malformation syndrome: fetal and placental pathology. Teratology. 1984;29:11-19.
12. Bamforth JS. Amniotic band sequence: Streeter’s hypothesis re-examined. Am J Med Genet. 1992;44:280-287.
13. Streeter GL. Focal deficiencies in fetal tissues and their relation to intrauterine amputations. Contrib Embryol Carnegie Inst. 1930;22:1-44.
14. Moerman P, Fryns JP, Vandenberghe K, et al. Constrictive amniotic bands, amniotic adhesions, and limb–body wall complex: discrete disruption sequence with pathogenetic overlap. Am J Med Genet. 1992;42:470-479.
15. Van Allen MI, Curry C, Gallagher L. Limb body wall complex. I. Pathogenesis. Am J Med Genet 1987;28:529-548.
16. Miller ME, Graham JM Jr, Higginbottom MC, et al. Compression-related defects from early amnion rupture: evidence for mechanical teratogenesis. J Pediatr. 1981;98:292-297.
17. Lubinsky M, Sujansky E, Sanders W, et al. Familial amniotic bands. Am J Med Genet. 1983;14:81-87.
18. Ho DM, Liu HC. The ABS: report of two autopsy cases and review of the literature. Clin Med J. 1987;39:429-436.
19. Hukki J, Balan P, Ceponiene R, et al. A case study of amnion rupture sequence with acalvaria, blindness, and clefting: clinical and psychological profiles. J Craniofac Surg 2004; 15:185.
20. Huang CC, Eng HL, Chen WJ. Amniotic band syndrome: report of two autopsy cases. Chang Gung Med J. 1995;18:371-377.
21. Sakiyama T, Umegaki-Arao N, Sasaki T, et al. Case of dominant dystrophic epidermolysis bullosa with amniotic band syndrome. J Dermatol 2017; 44:102.
22. Young ID, Lindenbaum RH, Thompson EM, et al. Amniotic bands in connective tissue disorders. Arch Dis Child. 1985;60:1061-1063.
23. Marras A, Dessì C, Macciotta A. Epidermolysis bullosa and amniotic bands. Am J Med Genet 1984; 19:815.
24. Rujiwetpongstorn J, Tongsong T. Amniotic band syndrome following septostomy in management of twin-twin transfusion syndrome: a case report. J Perinatol 2008; 28:377.
25. Han M, Afshar Y, Chon AH, et al. Pseudoamniotic Band Syndrome Post Fetal Thoracoamniotic Shunting for Bilateral Hydrothorax. Fetal Pediatr Pathol 2017; 36:311.
26. Winer N, Salomon LJ, Essaoui M, et al. Pseudoamniotic band syndrome: a rare complication of monochorionic twins with fetofetal transfusion syndrome treated by laser coagulation. Am J Obstet Gynecol 2008; 198:393.e1.
27. Iqbal CW, Derderian SC, Cheng Y, et al. Amniotic band syndrome: a single-institutional experience. Fetal Diagn Ther 2015; 37:1.
28. Weinstein B, Hassouba M, Flores RL, et al. Digital-Facial Translocation in Amniotic Band Sequence: Evidence of the Intrinsic Theory. J Craniofac Surg 2018; 29:1890.
29. Fiedler JM, Phelan JP. The amniotic band syndrome in monozygotic twins. Am J Obstet Gynecol 1983; 146:864.
30. Rehder H. Fetal limb deformities due to amniotic constrictions (a possible consequence of preceding amniocentesis) Pathol Res Pract 1978; 162:316-326
31. Lage JM, VanMarter LJ, Bieber FR. Questionable role of amniocentesis in the etiology of amniotic band formation: a case report. J Reprod Med. 1988;33:71-73.
32. Stanek J, de Courten-Myers G, Spaulding AG, et al. Case of complex craniofacial anomalies, bilateral nasal proboscides, palatal pituitary, upper limbs reduction, and amnion rupture sequence: disorganization phenotype? Pediatr Dev Pathol 2001; 4:192.
33. Chemke J, Gaff G, Hurwitz N, et al. The ABS. Obstet Gynecol. 1973;41: 332-336.
34. Daly CA, Freeman J, Weston W, et al. Prenatal diagnosis of ABS in a methadone user: review of the literature and a case report. Ultrasound Obstet Gynecol. 1996;8:123-125.
35. Weinzweig N, Barr A. Radial, ulnar, and median nerve palsies caused by a congenital constriction band of the arm: single-stage correction. Plast Reconstr Surg 1994; 94:872.
36. Kaufman AJ, Fleischer AC, Thieme GA, et al. Separated chorioamnion and elevated chorion: sonographic features and clinical significance. J Ultrasound Med. 1985;4:119-125.
37. Martínez-Frías ML. Epidemiological characteristics of amniotic band sequence (ABS) and body wall complex (BWC): are they two different entities? Am J Med Genet 1997; 73:176.
38. Barzilay E, Harel Y, Haas J, et al. Prenatal diagnosis of amniotic band syndrome – risk factors and ultrasonic signs. J Matern Fetal Neonatal Med 2015; 28:281.
39. Graf JL, Bealer JF, Gibbs DL, et al. Chorioamniotic membrane separation: a potentially lethal finding. Fetal Diagn Ther. 1997;12:81-84.
40. Heifetz SA. Strangulation of the umbilical cord by amniotic bands. Pediatr Pathol. 1984;2:285-304
41. Czichos E, Lukaszek S, Krekora M, et al. Early amnion rupture and fetal and newborn defects as an obstetrical and pathomorphological problem. Ginekol Pol. 2005;76:448-456.
42. Ribeiro MG, Castilla EE, Orioli IM. Can amputated digits point to clues about etiology? Am J Med Genet A. 2004;128A:93-94.
43. Sentilhes L, Verspyck E, Eurin D, et al. Favorable outcome of tight constriction band secondary to amniotic band syndrome. Prenat Diagn. 2004;24:198-201.
44. Dyson RL, Pretorius DH, Budorick NE, Johnson DD, Sklansky MS, Cantrell CJ, et al. Three-dimensional ultrasound in the evaluation of fetal anomalies. Ultrasound Obstet Gynecol. 2000;16:321–8.
45. Chen CP. Prenatal diagnosis of limb body wall complex with craniofacial defects, amniotic bands, adhesions and upper limb deficiency. Prenat Diagn. 2001;21:418-424.
46. Chen CP, TY Chang, YH Lim, et al. Prenatal sonographic diagnosis of acrania associated with amniotic bands. J Clin Ultrasound. 2004;32:256-260.
47. Sauerbrei E, Cooperberg PL, Poland BJ. Ultrasound demonstration of the normal fetal yolk sac. J Clin Ultrasound. 1980;8:217-220.
48. Burrows PE, Lyons EA, Phillips HJ, et al. Intrauterine membranes: sonographic findings and clinical significance. J Clin Ultrasound. 1982;10:1-8.
49. Patten RM, VanAllen MI, Mack LA, et al. Limb–body wall complex: in utero sonographic diagnosis of a complicated fetal malformation. AJR Am J Roentgenol. 1986;146:1019-1024.
50. Spirit BA, Kagan EH, Rozanski RM. Abruptio placenta: sonographic and pathologic correlation. AJR Am J Roentgenol. 1979;133:877-881.
51. Filly RA, Golbus MS. The fetus with amniotic band syndrome. In: Harrison MR, Golbus MS, Filly RA, eds. The Unborn Patient. 2nd ed. Philadelphia, PA: WB Saunders; 1991:440-447.
52. Asherman JG. Amenorrhoea traumatic. Br J Obstet Gynaecol. 1948;55: 23-27.
53. Comninos AC, Zourlas PA. Treatment of intrauterine adhesions (Asherman’s syndrome). Am J Obstet Gynecol. 1969;105:862-867.
54. Mahony BS, Filly RA, Callen PW, et al. The amniotic band syndrome: antenatal diagnosis and potential pitfalls. Am J Obstet Gynecol. 1985; 152:63-68.
55. Randal SB, Filly RA, Callen PW, et al. Amniotic sheets. Radiology. 1988;166:633-638. preceding amniocentesis). Pathol Res Pract. 1978;162:316-326.
56. Tan JV, et al. The amniotic sheet: a truly benign condition? Ultrasound Obstet Gynecol 2005; 26:639.
57. Rohrbach M, Chitayat D, Drake J, et al. Prenatal diagnosis of fetal exencephaly associated with amniotic band sequence at 17 weeks of gestation by fetal magnetic resonance imaging. Fetal Diagn Ther 2007; 22:112.
58. Neuman J, Calvo-Garcia MA, Kline-Fath BM, et al. Prenatal imaging of amniotic band sequence: utility and role of fetal MRI as an adjunct to prenatal US. Pediatr Radiol 2012; 42:544.
59. Rypens F, Metens T, Rocourt N, Sonigo P, Brunelle F, Quere MP, Guibaud L, Maugey-Laulom B, Durand C, Avni FE, Eurin D. Fetal lung volume: estimation at MR imaging-initial results. Radiology. 2001 Apr;219(1):236-41.
60. Meyers ML, Garcia JR, Blough KL, Zhang W, Cassady CI, Mehollin-Ray AR. Fetal Lung Volumes by MRI: Normal Weekly Values From 18 Through 38 Weeks’ Gestation. AJR Am J Roentgenol. 2018 Aug;211(2):432-438.
61. Nelson LD, Grobman WA. Obstetric morbidity associated with amniotic sheets. Ultrasound Obstet Gynecol 2010; 36:324.
62. Tuuli MG, Shanks A, Bernhard L, et al. Uterine synechiae and pregnancy complications. Obstet Gynecol 2012; 119:810.
63. ten Donkelaar HJ, Hamel BC, Hartman E, et al. Intestinal mucosa on top of a rudimentary occipital meningocele in amniotic rupture sequence: disorganization-like syndrome, homeotic transformation, abnormal surface encounter or endoectodermal adhesion? Clin Dysmorphol 2002; 11:9.
64. Wilcox WR, Coulter CP, Schmitz ML. Congenital limb deficiency disorders. Clin Perinatol 2015; 42:281.
65. Lopriore E, Lewi L, Oepkes D, et al. In utero acquired limb ischemia in monochorionic twins with and without twin-to-twin transfusion syndrome. Prenat Diagn 2008; 28:800.
66. Javadian P, Shamshirsaz AA, Haeri S, et al. Perinatal outcome after fetoscopic release of amniotic bands: a single-center experience and review of the literature. Ultrasound Obstet Gynecol 2013; 42:449.
67. Bollard HO, Shook L, Lain KY, Burns L, Crombleholme TM. An embolic complication with severe twin-twin transfusion syndrome after fetoscopic intervention. J Perinatology 29; 250-251, 2009
68. Hill L, Kislak S, Jones N. Prenatal ultrasound diagnosis of a forearm constrictive band. J Ultrasound Med 1988;7: 293–295.
69. Kanayama MD, Gaffey TA, Ogburn PL Jr. Constriction of the umbilical cord by an amniotic band, with fetal compromise illustrated by reverse diastolic flow in the umbilical artery: a case report. J Reprod Med. 1995;40:71-73.
70. Adzick NS, Sutton L, Crombleholme TM, et al. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675-1676.
71. Crombleholme TM, Robertson FM, Marx G, et al. Fetoscopic cord ligation to prevent neurologic injury in monozygous twins. Lancet. 1996;348:191.
72. Crombleholme TM, Dirkes K, Whitney TM, et al. Amniotic band syndrome in fetal lambs. I. Fetoscopic release and morphometric outcome. J Pediatr Surg. 1995;30:974-978.
73. Quintero RA, Morales WJ, Phillips J, et al. In utero lysis of amniotic bands. Ultrasound Obstet Gynecol. 1997;10:316-320.
74. Tadmor OP, Kreisberg GA, Achiron R, et al. Limb amputation in amniotic band syndrome: serial ultrasonographic and Doppler observations. Ultrasound Obstet Gynecol. 1997;10:312-315.
75. Crombleholme TM. The fetus with amniotic band syndrome, the unborn patient. In: Harrison MR, Evans MI, Adzick NS, Holzgreve W, eds. The Art and Science of Fetal Therapy. 3rd ed. Philadelphia, PA: WB Saunders; 2001:489-502.
76. Keswani SG, Johnson MP, Adzick NS, et al. In utero limb salvage: fetoscopic release of amniotic bands for threatened limb amputation. J Pediatr Surg. 2003;38:848-851.
77. Ronderos-Dumit D, Briceño F, Navarro H, Sanchez N. Endoscopic release of limb constriction rings in utero. Fetal Diagn Ther 2006; 21(03):255–258
78. Soldado F, Aguirre M, Peiró JL, et al. Fetoscopic release of extremity amniotic bands with risk of amputation. J Pediatr Orthop 2009;29(03):290–293
79. Peiró JL, Carreras E, Soldado F, et al. Fetoscopic release of umbilical cord amniotic band in a human fetus. Ultrasound Obstet Gynecol 2009;33(02):232–234
80. Hüsler MR, Wilson RD, Horii SC, et al. When is fetoscopic release of amniotic bands indicated? Review of outcome of cases treated in utero and selection criteria for fetal surgery. Prenat Diagn 2009; 29:457.
81. Snyder C, Lang L, Lewis D, Habli M, Lim FY, Crombleholme TM: Fetoscopic release of bands in amniotic band syndrome: Outcomes in operative and nonoperative candidates. Am J Obstet Gynecol 2011; S160
82. Richter J, Wergeland H, DeKoninck P, De Catte L, Deprest JA. Fetoscopic release of an amniotic band with risk of amputation: case report and review of the literature. Fetal Diagn Ther 2012; 31(02):134–137
83. Belfort MA, Whitehead WE, Ball R, et al. Fetoscopic Amniotic Band Release in a Case of Chorioamniotic Separation: An Innovative New Technique. AJP Rep 2016; 6:e222.50.
84. Ting YH, Lao TT, Law KM, et al. Pseudoamniotic Band Syndrome after In Utero Intervention for Twin-to-Twin Transfusion Syndrome: Case Reports and Literature Review. Fetal Diagn Ther 2016; 40:67
85. Abdel-Sattar M, Chon A, Chen B, et al. Salvage of Necrotic-Appearing Limb after In Utero Endoscopic Lysis of Constriction Bands. AJP Rep 2017; 7:e74.
86. Gueneuc A, Chalouhi GE, Borali D, Mediouni I, Stirnemann J, Ville Y: Fetoscopic Release of Amniotic Bands Causing Limb Constriction: Case Series and Review of the Literature. Fetal Diagn Ther 2019;46:246–256
87. Rezai S, Faye J, Chadee A, et al. Amniotic Band Syndrome, Perinatal Hospice, and Palliative Care versus Active Management. Case Rep Obstet Gynecol 2016; 2016:9756987.
88. Levy R, Lacombe D, Rougier Y, Camus E. Limb body wall complex and amniotic band sequence in sibs. Am J Med Genet A 2007; 143A:2682.
89. Findik H, Malkoc C, Uzunismail A. Long-term effects of amniotic bands not treated at an early age. Plast Reconstr Surg. 2006;117: 713-714.
90. O’Driscoll M, Peckham C, Kerr B. Four limb syndactyly, constriction rings and skin tags; amniotic bands or disorganization-like syndrome. Clin Dysmorphol 2008; 17:255.
91. Jensen KK, Oh KY, Kennedy AM, Sohaey R. Intrauterine Linear Echogenicities in the Gravid Uterus: What Radiologists Should Know. Radiographics 2018; 38:642.
92. Robin NH, Franklin J, Prucka S, et al. Clefting, amniotic bands, and polydactyly: a distinct phenotype that supports an intrinsic mechanism for amniotic band sequence. Am J Med Genet A 2005; 137A:298.
93. Zionts LE, Osterkamp JA, Crawford TO, Harvey JP Jr. Congenital annular bands in identical twins. A case report. J Bone Joint Surg Am 1984; 66:450.
94. Bodamer OA, Popek EJ, Bacino C. Atypical presentation of amniotic band sequence. Am J Med Genet 2001; 100:100.