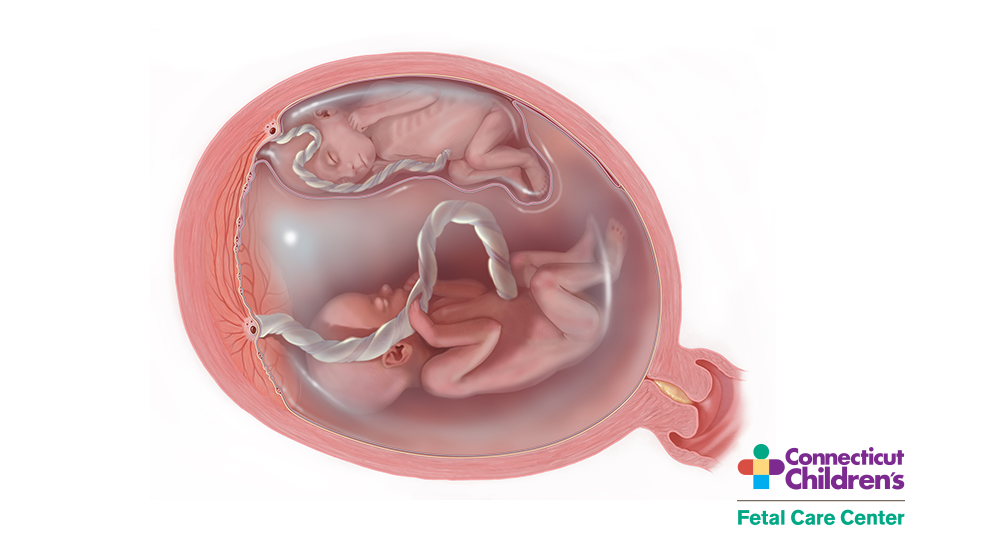
Twin-Twin Transfusion Syndrom (TTTS) is a rare, life-threatening clinical syndrome that only occurs in identical twins or higher order multiple gestations that share a placenta. TTTS is characterized by one twin developing excessive amniotic fluid volume (polyhydramnios) and the co-twin too little amniotic fluid (oligohydramnios). (The twin with too little amniotic fluid is referred to as the donor twin.)
While the exact cause of TTTS is not known, it is associated with vascular connections on the surface of the placenta and a release of vasoactive mediators by the placenta, which have differential effects on the twins.
Although TTTS can occur at any time during pregnancy, even while a mother is in labor at term, it most often arises in the second trimester of pregnancy. When it appears early in pregnancy (around 12-26 gestational weeks), it is known as chronic TTTS. These cases are the most serious because the babies are generally too immature to be delivered. TTTS is also progressive; more time in the womb affects the abnormalities present with TTTS. Without treatment, babies affected by chronic TTTS are the most likely to not survive or to have severe handicaps or birth defects.
Acute TTTS describes those cases that occur suddenly, when the blood pressures between the twins begin to vary greatly. This may occur in labor at term or during the last trimester of pregnancy. Acute TTTS twins may have a better chance to survive because of their advanced gestational age, but are also at risk for developing handicaps.
Over 65% of pregnancies affected by TTTS will also have evidence of selective fetal growth restriction (FGR), most often in the donor twin. FGR may have a significant impact on the prognosis and risk of handicap. Another condition seen in identical twins, because they share a placenta and have vascular connections between the twins, is the twin anemia polycythemia sequence (TAPS). Unlike chronic TTTS, in which there is no transfusion from one twin to the other, in TAPS there is a slow progressive transfusion from one twin to the other resulting in one twin becoming anemic and the other twin becoming polycythemia (too much blood). TAPS is diagnosed by differences in the peak systolic middle cerebral artery flow velocities on Doppler ultrasound, which go up in the anemic baby and go down in the polycythemic baby
Dr. Crombleholme’s extensive experience caring for monochorionic (identical) twin pregnancies enables us to differentiate between conditions such as TTTS, FGR, and TAPS, all of which may be present in the same pregnancy and vary in severity. Each condition requires close monitoring and treatment by a skilled fetal care center team.
Features of twin-twin transfusion syndrome include twins of the same gender, an obvious size difference between the twins due to unequal sharing of the placenta, and a difference in the volume of amniotic fluid surrounding the two fetuses.
One twin (the donor twin) has very little fluid (oligohydramnios) and is volume-constricted with little urine output. The co-twin (called the recipient) has an expanded blood volume causing the right atrium to release brain-type atrial natriuretic peptide (BNP) to stretch, which is one of the most powerful diuretic agents. This results in excessive urination, an enlarged bladder, and polyhydramnios. The volume overload in the recipient twin causes the circulatory system to become overloaded, causing thickening and dysfunction of the heart and prenatal heart failure, eventually resulting in swelling of soft tissues and fluid collection in the chest and abdomen (hydrops).
The donor twin, who is volume-restricted, may stop producing urine at all. This may result in a small amniotic sac and a small or absent bladder. Without fetal surgeries and interventions, the condition can be fatal for both twins and associated with severe neurodevelopmental abnormalities if a twin survives. As the signs of TTTS progress, the mother’s health may be compromised. As the recipient twin tries to deal with excess fluid retention by creating more and more amniotic fluid, the mother’s uterus may become overstretched. This may not only cause the mother discomfort, but can cause preterm labor, put pressure on the lower part of the uterus, and shorten the cervical length. The cervix may open, or the membranes may rupture from the continuing pressure, resulting in miscarriage or preterm delivery. This often occurs at an early gestational age before viability when the chances for survival are poor.
It is important for mothers who are diagnosed with monochorionic twins to be closely monitored for possible signs of TTTS.
Outcomes of Selective Fetoscopic Laser Photocoagulation for TTTS
Dr. Crombleholme has been a pioneer in the treatment of TTTS using selective fetoscopic laser photocoagulation for over 25 years and has performed over 1400 fetal surgical procedures making him one of the most experienced fetal surgeons in the world.
Would you like to schedule an appointment with our Fetal Care Center?
For Medical Professionals
Twin-twin transfusion syndrome (TTTS) is a complication of monochorionic multiple gestations in which the circulation of one twin communicates with the circulation of the other twin. Acute clinically relevant TTTS is rare and usually occurs during delivery. It is associated with an acute hemodynamic incident, such as cord compression or rupture of a vessel as in vasa previa (1,2). In these instances, the twins are usually of similar birth weight, but due to acute shift of blood through large chorionic plate vascular anastomoses, usually veno-venous, one twin is pale and the other twin is plethoric.
Chronic TTTS is a condition usually presenting in the second or third trimester. It’s thought to be a condition of sustained net imbalance of blood volume transfused from one twin to the other across the monochorionic placenta via intertwin vascular anastomoses. By convention, this results in one twin (referred to as the donor twin) becoming hypovolemic with reduced urine output and reduced amniotic fluid volume. The recipient twin is usually hypervolemic, with polyhydramnios and cardiac changes of hypertrophic cardiomyopathy. TTTS is thought to complicate between 10% and 15% of all monochorionic pregnancies but there is a broad range of reported incidences ranging from 4% to 35% of monochorionic gestations in the United States (3). This broad range is thought to be due to differences in clinical criteria used to make the diagnosis (4). Severe TTTS is thought to complicate between 5% and 17.5% of cases of monochorionic gestations (5).
TTTS is a clinical syndrome that is diagnosed sonographically with differences in amniotic fluid volume between twins, the so-called “poly-oli” or twin oligohydramnios polyhydramnios syndrome. In severe TTTS, the donor twin becomes progressively hypovolemic developing oliguria and oligohydramnios due to decreased renal perfusion often accompanied by characteristic abnormal umbilical artery Doppler velocimetry changes. The oligohydramnios, or anhydramnios, may restrict donor fetal movement. As the donor twin becomes more closely enwrapped in its own amnion, it appears “ stuck” to the uterine sidewall or placenta (i.e., “stuck twin” syndrome). In contrast, the recipient twin becomes hypervolemic and hypertensive leading to polyhydramnios with cardiac hypertrophy and may exhibit abnormal venous Doppler studies due to progressive ventricular hypertrophy and worsening diastolic compliance. Both twins are adversely affected in TTTS and hydrops fetalis can develop in either twin. In the recipient twin, this is due to progressive congestive heart failure. If left untreated, severe TTTS has an 80 to 100% mortality rate, particularly if it is detected before 20 weeks gestation (6,7).
Prior to the development of ultrasonography, the criteria for diagnosing TTTS were derived from neonatal experience with the condition in which a hemoglobin difference of > 5 g/dL in neonatal hemoglobin value and > 20% discordance in weight was seen between the twins. These classic discrepancies between weight and hemoglobin are now appreciated to occur in fewer than 25% of cases detected by ultrasound (8,9).
The diagnosis of TTTS is one of exclusion made on the basis of amniotic fluid discordance in which the recipient twin has a deepest vertical pocket (DVP) of > 8 cm, and the donor twin has a DVP of < 2 cm (10). One must be careful, however, to exclude conditions that may mimic TTTS, including selective intrauterine growth restriction (sIUGR), viral infection and preterm premature rupture of membranes. TTTS can be complicated by sIUGR in up to 65% of cases, which may present a challenge in distinguishing isolated sIUGR from TTTS complicated by sIUGR. In isolated sIUGR, the unaffected twin is likely to have normal amniotic fluid volume while the affected twin may have olighydramnios or even anhydramnios. There may be discordant amniotic fluid volumes between the two sacs but the DVP values must meet the diagnostic criteria in order to make a diagnosis of TTTS. A caveat to the diagnostic criteria are those cases presenting prior to 18 weeks gestation in which a DVP > 6 cm is considered diagnostic of TTTS when the donor sac DVP is < 2 cm. In addition to discordance in amniotic fluid volumes, the twins may show characteristic Doppler velocimetry changes. While not diagnostic, these changes are consistent with the diagnosis of TTTS, which includes absent end diastolic umbilical artery flow in the donor umbilical artery, loss or reversal of the wave of the ductus venosus, and venous pulsations of the umbilical vein. These are not specific to TTTS and can be seen in other conditions as well.
There is a spectrum of severity of TTTS. Amniotic fluid discordance may be present but may not meet the strict criteria for TTTS (i.e., a DVP in the recipient sac of 7 cm and a DVP in the donor sac of 3 cm). We would characterize this presentation as possible “incipient TTTS” as the strict amniotic fluid criteria have not yet been met. Confidence that we are dealing with TTTS, albeit in an incipient form, can be obtained from fetal echocardiographic assessment. Even in incipient TTTS, there are often findings on the fetal echocardiogram in the recipient's heart consistent with TTTS cardiomyopathy. The most common observations are abnormal compliance of the right ventricle with the fusion of the A and E waves giving a monophasic inflow pattern. In addition, mildly elevated myocardial performance index (Tei Index) values may be observed in the right ventricle of the recipient (11). These values may be at the upper limits of normal to mildly abnormal (i.e., 0.4 to 0.52). In early cases of TTTS, the left ventricle is almost never involved, and significant hypertrophy or valvular incompetence is rarely seen.
The lack of agreement on specific diagnostic criteria to define mid-gestation TTTS and the influence of older neonatal criteria have hampered understanding of its pathophysiology and slowed the development of more effective treatment strategies. The donor twin is characterized by oliguria, oligohydramnios or anhydramnios, growth restriction and abnormal umbilical artery Doppler velocimetry. The recipient, on the other hand, is characterized by polyuria, polyhydramnios, abnormal venous Dopplers, cardiac enlargement/failure, and eventually hydrops.
Clinicians caring for women with monochorionic pregnancies should have a strong clinical suspicion for TTTS. Sonographic signs of monochorionic diamniotic twins include a single placenta, a thin dividing membrane, a “T”-sign, and gender concordance. Before ruling out monochorionic diamniotic twins in the case when no dividing membrane is seen, a diligent search for a thin membrane tightly wrapped around one twin should be performed. The following findings are suggestive of the diagnosis of TTTS (meeting all sonographic criteria is not necessary for a diagnosis):
- Discrepancy in amniotic fluid between the amniotic sacs with polyhydraminos of one twin (largest vertical pocket greater than 8 cm) and oligohydraminos of the other (largest vertical pocket less than 2 cm)
- Discrepancy in size of the umbilical cords
- Presence of cardiac dysfunction in the polyhydramniotic twin
- Characteristically abnormal umbilical artery or ductus venosus Doppler velocimetry
- Less specifically, significant growth discordance (often > 20%). Though criteria for TTTS diagnosed in utero were initially derived from neonatal criteria relying on discordant weights (usually > 20%) and hemoglobin levels (usually difference of > 5 g/dL) between the twins, subsequent literature demonstrates that hemoglobin discordance is often not present in mid-gestation and advanced TTTS may be present before the threshold of 20% weight discordance is reached.
In addition to careful sonographic follow-up, mothers of monochorionic diamniotic twins should be alerted that rapid uterine growth, premature contractions and dyspnea may be symptoms of polyhydraminos. The differential diagnosis of TTTS includes uteroplacental insufficiency, growth disturbances due to abnormal cord insertions, intrauterine infection, preterm premature rupture of membranes of one twin, and discordant chromosomal or structural anomalies of one twin.
Quintero et al. (3) proposed a staging system for TTTS that considers a sequence of progressive sonographic features. The individual stages are described as follows:
- Stage I: Polyhydraminos in the recipient, severe oligohydraminos in donor but urine visible within the bladder in the donor
- Stage II: Polyhydraminos in the recipient, a stuck donor, urine not visible within the donor’s bladder
- Stage III: Polyhydraminos and oligohydraminos as well as critically abnormal Dopplers (at least one of: absent or reverse end diastolic flow in the umbilical artery, reverse flow in the ductus venosus, or pulsatile umbilical venous flow) with or without urine visualized within the donor’s bladder
- Stage IV: Presence of ascites or frank hydrops (fluid collection in two or more cavities) in either donor or recipient
- Stage V: Demise of either fetus. This staging system was descriptive but had not been validated as prognostically important.
Taylor et al (4) applied the Quintero staging system to a population treated with serial amnioreduction, septostomy and selective reduction alone or in combination. Taylor et al. found no significant influence of staging at presentation with survival in his conservatively treated group. Survival was significantly poorer where stage increased rather than decreased. These authors concluded that the Quintero staging system should be used with caution for determining prognosis at the time of diagnosis, but may be better suited for monitoring disease progression. A subsequent larger study from the same institution, however, showed that Quintero stage at presentation, at first treatment and at worst stage did in fact predict both perinatal and double survival but not survival of any twin (4). Duncombe et al. also showed a correlation of Quintero stage at initial presentation and perinatal survival (10).
The Quintero staging system provides a useful shorthand to describe the progression of TTTS along a spectrum of severity. However, it has potential limitations in its use in guiding therapy. In patients who present at Stage I with only amniotic fluid discordance, it may be difficult to know with certainty if they actually have TTTS. Patients with Stage II are usually thought to be only in the early stages of the disease. The largest group of patients tends to fall into Stage III. This stage, however, comprises a very broad spectrum of severity. At one end are patients whose only hemodynamic derangement is abnormal Doppler velocimetry, and at the other end of the spectrum are patients in whom the recipient twin has severe twin-twin cardiomyopathy. The latter patients may be premorbid without the development of hydrops (which would be Stage IV disease).
We have used fetal echocardiographic assessment of the recipient twin to stage these patients. This is in keeping with the view that, fundamentally, TTTS is a hemodynamic derangement. Fetal echocardiograms can distinguish degrees of severity amongst Stage III TTTS. Echocardiographic features include the presence and severity of atrioventricular valvular incompetence, ventricular wall thickening, and ventricular function as assessed by the Tei index (3,11). In a small series of cases of TTTS, 64% Quintero Stage II patients had significant TTTS cardiomyopathy and were upstaged to Stage III disease based on echocardiographic findings (5). The upstaging of patients from Stage I or II to Stage III may influence counseling regarding treatment options. These echocardiographic features are also used to assess response to therapy.
If a patient is initially only observed or treated with a trial of a single amnioreduction, fetal echocardiography can be used to detect the progression of TTTS and used as an indication to move on to selective fetoscopic laser photocoagulation (8).
The recognition that TTTS cardiomyopathy was a fundamental component of the pathophysiology of TTTS has led others to propose staging systems based on fetal echocardiographic findings. Rychik et al developed the CHOP Cardiovascular Profile Score (CHOP-CVPS) comprised of 12 parameters yielding a score ranging from 0 to 20 (15). The higher the score the worse the cardiac function. The CHOP-CVPS is difficult to establish because it requires an experienced fetal echocardiographer and considerable time to complete the assessment. In studies that have compared the CHOP-CVPS to the Quintero staging system, a positive correlation between rising CHOP-CVPS and Quintero stage was observed.
One of the most consistently observed factors in recipient cardiac assessment found to correlate with outcome is the Tei myocardial performance index (MPI). The Tei MPI is an essential component of the Cincinnati staging system. The increase in MPI in early Quintero stage TTTS provides a more nuanced ability to identify pregnancies at risk for consideration for fetal surgery. We have identified 55% of Quintero stage I and 65% of Quintero stage II will have significantly elevated Tei myocardial performance indices (5).
Selective fetal growth restriction (sFGR) affects 12 to 25% of monochorionic pregnancies (21). There is no consensus on a definition of selective fetal growth restriction and monochorionic twins but recent studies have focused on estimated fetal weight (22-25). Different thresholds have been suggested to establish a diagnosis of selective FGR from < 10th percentile of estimated fetal weight with or without associated umbilical artery Doppler abnormalities of absent or reversed diastolic flow. Alternatively, < 3rd percentile of estimated fetal weight, again with or without associated absent or reversed diastolic flow in the umbilical artery waveform, has also been suggested to be even more selective. Abdominal circumference and/or the degree of fetal weight discordance have also been used to diagnose sFGR (22-28). Selective FGR is an important complication of monochorionic pregnancies with a high rate of adverse neurodevelopmental outcomes and intrauterine fetal demise (22-24, 27–29). Because of the presence of inter-twin vascular connections in monochorionic twins, many have focused on the pattern of umbilical artery Doppler velocimetry in selective FGR (21). Fetuses with positive umbilical artery diastolic flow, are generally thought to have a favorable prognosis. Fetuses with persistently absent or reversed end-diastolic flow are thought to be at high risk for deterioration and intrauterine fetal demise (23, 26, 33). Those fetuses with intermittent absent or reversed diastolic flow, likely from transmitted waveforms from one twin to the other twin via large artery-to-artery placental anastomoses, are thought to be at the highest risk for acute intrauterine fetal demise (31, 32).
Gratacos et al., have proposed categorizing selective FGR in monochorionic gestations based on the appearance of the Doppler velocimetry of the umbilical artery into three types (21). In type I, there is positive end-diastolic flow in the umbilical artery. In general, these fetuses have the best prognosis. In type II, absent or reversed diastolic flow is constantly observed. These fetuses typically show a slow progression in the severity of selective FGR during the pregnancy. Lastly, in type III, there is intermittent absent or reversed diastolic flow alternating with positive diastolic flow over a short period of time. Type III fetuses are the most at risk for an acute intrauterine fetal demise thought to be secondary to the presence of large artery-to-artery vascular connections on the chorionic plate.
Twin anemia polycythemia sequence (TAPS) is an uncommon complication of monochorionic gestations that occurs as a result of small vascular connections between the twins on the chorionic plate, allowing chronic transfusion of blood from one twin to the other (1). The result is an anemic donor and a polycythemic recipient. TAPS can occur spontaneously and can develop concomitantly with TTTS and/or sFGR. The most common presentation is after fetoscopic laser treatment of TTTS due to residual vascular communication. The true history can vary from completely normal twins to severe cerebral injury to intrauterine fetal demise of one or both twins (33-36).
The incidence of spontaneous TAPS ranges from 1 to 5% of monochorionic twins, but may be more common as a complication following fetoscopic laser treatment of twin-twin transfusion syndrome ranging from 1 to 16% (33, 35, 37).
The diagnosis of TAPS is based on detecting increased peak systolic flow velocity in the middle cerebral artery (PSV-MCA) indicating the presence of fetal anemia in the donor twin. In contrast, the recipient has a decreased PSV-MCA indicating polycythemia (33). Other sonographic features of TAPS include cardiomegaly, evidence of cardiac dysfunction, and a “starry sky” appearance of the liver due to increased echogenicity of the portal vein kneels and decreased echogenicity of the liver parenchyma (38,39).
Diagnostic criteria for TAPS have traditionally used the PSV-MCA value of > 1.5 MoM in the donor combined < 1.0 MoM in the recipient. However, Tolenaar et al., suggested that the delta between the donor and recipient PSV-MCAs of > 0.5 MoM had greater diagnostic accuracy in TAPS (40). A Delphi consensus panel convened to standardize the diagnosis of TAPS suggested a cutoff value > 1.5 MoM in the donor and < 0.8 MoM in the recipient or a delta PSV-MCA of > 1.0 should be used (41).
Staging systems have been proposed to help guide the management of TAPS. The first such staging system was the Leiden staging which defined stage I as the donor having a PSV-MCA >1.5 MoM and the recipient having a PSV-MCA < 1.0 MoM. In stage II, the donor had a PSV-MCA of >1.7 MoM and the recipient < 0.8. In stage III, there is evidence of cardiac dysfunction in the donor twin with either stage I or II findings. In stage IV, there is evidence of hydrops in either twin, and in stage V, there has been the demise of one or both twins. A modification of this staging system has been suggested substituting delta PSV-MCA values in stages I and II. In stage I, a delta PSV-MCA of > 0.5, and for stage II > 0.7 with stages III through V being the same as the Leiden staging system.
A large multicenter trial of expectant management allowed a comparison of expectant management and fetoscopic laser photocoagulation on the duration of pregnancy in TAPS (42-44). Spontaneous resolution of TAPS was observed in 16% of cases. In 12% percent of expectantly managed cases, further treatment was indicated due to signs of TAPS progression.
There is currently no consensus for the treatment of TAPS and interventions range from purely expectant management, to early delivery if sufficiently far enough along in pregnancy, to intrauterine transfusion, to fetoscopic laser surgery, and lastly selective termination. Which of these treatment options is indicated is often determined by the severity of the TAPS and the gestational age at presentation. TAPS can present in combination with TTTS and/or sFGR and the severity of each of these diagnoses may determine the need for fetal intervention.
The twin-twin transfusion syndrome is a complication primarily of monochorionic multiple gestations mediated through vascular communications and having differential effects on the co-twins: one twin is hyperdynamic whereas the other is hypodynamic. The etiology of TTTS is unknown, but vascular connections on the placenta between twins are necessary for it to occur. The majority of monochorionic twins gestations have vascular anastomoses between the co-twins, although only a percentage, ranging from 4-17%, develop TTTS. Communications between the recipient and donor twin may be artery-to-artery (AA), vein-to-vein (VV), or most commonly artery-to-vein (AV) within a placental cotyledon. Depending on the number and type of anastomoses present, the exchange of blood may be balanced or unbalanced. Shifts in blood flow between the twins may be either acute, as in the case of co-twin demise, or chronic. A true transfusion from one twin to another is unusual in mid-gestation TTTS. Percutaneous umbilical blood sampling performed on twin pairs with TTTS has shown identical hemoglobin values in both twins (9).
AV anastomoses consist of a single unpaired artery carrying blood from one twin to a placental cotyledon and a single unpaired vein transporting blood from that cotyledon to the other twin. Most likely, AV anastomoses are primarily responsible for the exchange of blood and vasoactive mediators from the recipient to the donor twin (7). Bidirectional AA anastomoses, on the other hand, are thought to be protective and, if present in sufficient numbers, able to compensate for the AV-mediated intertwin transfusion: VV anastomoses may also be protective although likely shunt less blood due to a lower pressure differential. Recent evidence suggests that ultrasound detection of an AA anastomosis confers a survival advantage in TTTS independent of Quintero stage (6). Though the patients in the study of Tan et al. were treated with various methods including serial amnioreduction, septostomy, bipolar cord occlusion, and laser ablation, multiple logistic regression failed to show that first-line treatment modality predicted survival after correction for stage, AA anastomosis and disease progression (7). For stages I, II and III, detection of AA anastomosis predicted better perinatal (100% versus 63%, 100% versus 59%, 83% versus 44%, presence of AA anastomosis versus absence of AA anastomosis respectively) and double survival rates (100% versus 52%, 100% versus 46%, 78% versus 26%). These authors suggested the use of a modified staging system incorporated the presence or absence of an AA anastomosis.
There is mounting evidence, however, that the pathophysiology of TTTS is more complex than mere volume shifts between co-twins. For example, changes in the renin-angiotensin system compound the renal changes initiated by hemodynamic changes in the donor and recipient (6). Angiotensin II usually helps to compensate in the setting of volume depletion. In the setting of TTTS, however, the intrarenal vasoconstriction mediated by angiotensin II following the upregulation of renin synthesis and release may exacerbate oligohydraminos. Donor fetuses after demise have evidence of increased renin synthesis and renal tubular dysgenesis (11). While recipient fetuses demonstrate downregulation of renin expression, glomerular and arterial lesions in the kidneys are suggestive of hypertension-induced microangiopathy. These findings suggest that hypertensive changes in the recipient twin may be due to vascular shunting of renin from the donor (11). It is not clear whether alterations in the renin-angiotensin system are primary or secondary effects of TTTS.
Consistent with the hypothesis that hypertensive mediators play an important role in TTTS is the finding by Bajoria et al. of elevated levels of endothelin-1, a potent vasoconstrictor, in the serum of recipient twins 2.5-fold higher than in donor twin (45). Moreover, plasma endothelin-1 levels were significantly higher in the recipient twins with hydrops than those with mild or no hydrops (45). Endothelin-1 may also be important for the regulation of amniotic fluid volume, both by itself and in the pathway leading to higher human brain natriuretic peptide (BNP) levels in amniotic fluid (45). Both endothelin-1 and BNP amniotic fluid levels correlate with the amniotic fluid index in TTTS. Recipient twin amniotic fluid levels of endothelin-1 and BNP are the highest, followed by amniotic fluid levels from non-TTTS monochorionic twins, followed by amniotic fluid levels from donor twins (46).
We believe that high levels of vasoactive mediators are preferentially shunted to the recipient twin resulting in hypertension and hypertensive cardiomyopathy. Interruption of vascular communications by selective fetoscopic laser photocoagulation may eliminate the hypertensive stress in the recipient twin by preventing vasoactive mediators from crossing to the recipient. Consistent with this vasoactive mediator hypothesis, we have seen the development of hypertension and hypertensive cardiomyopathy in some donor twins following successful fetoscopic laser photocoagulation. Presumably, the vasoactive mediators are shunted toward the donor twin in these cases.
Cardiovascular compromise occurs in most recipient twins and is a major cause of death for these fetuses (6). In addition, cardiovascular disease in the recipient twin is a significant indirect contributor to morbidity and mortality in the donor co-twin. Echocardiographic examination of the twins is thus an essential component of the initial workup of twin-twin transfusion syndrome as well as follow-up evaluation for progression of the disease and response to treatment. In addition, the study of short-term and long-term cardiovascular effects of various therapeutic interventions is critical.
Recipient twins can develop progressive cardiomyopathy. Although both ventricular dilation and myocardial hypertrophy may occur, the latter predominates and typically only mild evidence of dilatation is seen (6,477). Usually, the right ventricle (RV) is compromised first and to a more significant degree than the left ventricle (LV) (46). In one study of 28 women with TTTS who received echocardiographic evaluation prior to any intervention, right ventricular and/or left ventricular hypertrophy was detected in 58% of recipient twins, and biventricular hypertrophy was observed in 33% of recipient twins (6). Biventricular diastolic dysfunction was present in two-thirds of recipient twins whereas right ventricular systolic dysfunction was present in 35% (9). Atrioventricular valve involvement is also common with moderate insufficiency reported in 71% of recipient twins with structurally normal hearts (48). Moderate to severe tricuspid and mitral regurgitation is more common in Quintero stage III and IV patients (6). Peak velocity of tricuspid and mitral regurgitant jets suggests the presence of ventricular hypertension in echocardiographic data from 39 recipient twins (48). Estimates of RV systolic pressure based on tricuspid regurgitant jet velocity are commonly elevated to 60 to 80 mm Hg, and pressures in excess of 100 mm Hg can be seen in severe cases.
Fetal echocardiography can also be used to assess the response of the recipient twin to fetoscopic laser surgery providing independent confirmation of successful treatment of the TTTS. Habli et al, have shown that in TTTS successfully treated by fetoscopic laser surgery that there is a >10% improvement in the Tei myocardial performance index on post-operative day 5 which only occurs if there has been a complete arrest of the TTTS (49). We routinely obtain a post-operative fetal echocardiogram to confirm that the TTTS has been arrested before sending the patient back to her referring doctor.
Several cases of acquired pulmonary atresia/stenosis with intact ventricular septum have been described in the recipient twin (6,50). In our own series, we have seen 50 such cases with varying degrees of pulmonary stenosis or pulmonary atresia (51). Worsening RV hypertrophy, reduced RV systolic function, and severe tricuspid regurgitation result in progressively diminished flow across the pulmonic valve, resulting in stenosis or atresia. In one such patient undergoing postnatal cardiac surgery, a structurally normal pulmonary valve with valve leaflet adhesion was found. These observations are not consistent with primary structural heart disease but rather acquired valvular atresia/stenosis related to TTTS, a unique form of “acquired congenital” heart disease.
Structural heart disease is much more common in monochorionic gestations with an incidence ranging from 8 to 10% (50). TTTS however, appears to be a rare condition that can cause an acquired form of structural heart disease affecting primarily the right ventricular outflow tract (RVOT). In a review of our experience with 610 cases of TTTS, Michelfelder et al., found an 8.7% incidence of RVOT abnormalities in the recipient twins but none in the donor twins (51). In this series, pulmonary atresia was most common (46%) followed by pulmonary stenosis (35%) and isolated pulmonary insufficiency (19%). While two-thirds of the recipient twins showed improvement of the RVOT abnormalities following successful fetoscopic laser surgery for the TTTS, a third persisted into postnatal life and often required balloon valvuloplasty. It has been suggested that these acquired structural lesions can progress even when function has improved, perhaps due to endothelial damage leading to the development of a dysplastic pulmonary valve (51).
The pathophysiology of RVOT abnormalities is thought to be due to altered flow in TTTS recipients with progressive dilation of the RV, diastolic dysfunction, and worsening atrioventricular valve incompetence . This reduces flow across the pulmonic valve and leads to pulmonic stenosis, atresia, or insufficiency.
Although structural heart disease in the donor twin in TTTS is less commonly observed prenatally, these babies appear to be at a greater risk of coarctation of the aorta. This is a difficult diagnosis to make prenatally (52). The mechanism of coarctation of the aorta in the donor twin is thought to be related to the relatively hypovolemic state, which results in reduced flow through the aorta and reduced growth of the aortic arch. Postnatally, there is an increased incidence of systolic and diastolic hypertension in both the donor and the recipient twin in TTTS (53). This appears to resolve with normalization of blood pressure by 10 years of age (54).
Cardiovascular changes in the donor twin are usually less dramatic. Myocardial changes are rare, and ventricular function and atrioventricular valve competence are usually preserved (19). In several series of patients, absent or reversed end diastolic flow was noted prior to treatment in 12-39% of donor twins (55-58). The abnormalities in umbilical artery velocimetry are reversible; among survivors after laser therapy, 27-30% showed a reappearance of end-diastolic flow within 24 hours post-operatively (57,58). Abnormal umbilical artery Dopplers are more common in donor than recipient twins.
If treatment (whether amnioreduction or laser photocoagulation therapy) is successful, there should be an arrest in the progression of TTTS cardiomyopathy and even a reversal of the existing disease. Progression of the cardiac findings suggests treatment failure either due to a lack of response to amnioreduction or to a missed vascular connection with fetoscopic laser treatment. Specifically, progressive changes noted after amnioreduction include worsening hypertrophy of the right or left ventricle or interventricular septum (6,59,60). In addition, in one report, RV systolic function stayed or became abnormal in the majority of patients (15/19 patients, 79%) whereas RV systolic function normalized in 1 recipient only (6). On the contrary, one small study reported improved cardiac function following amnioreduction (61) and another suggested altered progression of TTTS cardiomyopathy after laser photocoagulation (58). Finally, the discordance of pulse wave velocity in brachial arteries of survivors of TTTS is altered with a laser to be more similar to dichorionic controls, whereas the same alterations in vascular programming are not seen with survivors treated by non-laser methods (62). Increased pulse wave velocity reflects increased vascular stiffness. This study suggests that in utero vascular remodeling may be altered by definitive laser therapy.
While much attention has focused on the effect of treatment on survival in TTTS, the neurologic morbidity among survivors is often under-appreciated. Due to the shared placental circulation, if the death of one co-twin occurs, there is an acute fall in blood pressure causing the placental resistance to fall. This drop in resistance across the placental vascular connections can result in a decrease in cerebral perfusion pressure and ischemic injury in the brain of the surviving twin. Endoscopic evidence of feto-fetal hemorrhage from a recipient to donor twin occurs within three hours of the spontaneous demise of the donor; these authors noted endoscopic and middle cerebral artery Doppler evidence of paradoxical anemia in the recipient and polycythemia in the donor (90). Brain injury, however, can occur in TTTS even when both twins survive. In the recipient with both surviving twins, neurologic damage could be related to secondary polycythemia and venous stasis. In the donor, neurologic injury may be due to anemia and hypotension.
A few studies report longer-term neurodevelopmental outcomes. Importantly, survivors who develop neurologic handicaps and mental retardation do not always have abnormal neonatal ultrasonography. Similarly, not all children with abnormal ultrasounds have clinically significant neurodevelopmental deficits. In one small study that followed TTTS survivors for a mean of 6.2 years (range 4-11 years), the incidence of cerebral palsy was 26% (5/19 infants) in the group treated by serial amnioreduction (91). All of these children had abnormal mental development in addition to motor deficits. Of note, three of the five children had normal neonatal head ultrasounds. In the combined cohort of children whose mothers had been treated with amnioreduction or conservative treatment, 22% (5/23) of the children without cerebral palsy or abnormal mental development had mild speech delay and required special education. One limitation to this and other studies is the lack of a comparable conservatively treated cohort group. Given the improved survival of TTTS babies with amnioreduction and other treatment modalities, however, it is unlikely that we will ever have such a cohort for comparison.
Even fewer studies have examined the long-term outcome of survivors of TTTS treated with intrauterine laser photocoagulation therapy. Banek et al. reported that in 89 such children, 78% showed normal development at a median age of 22 months (92). Eleven percent had minor neurologic abnormalities including strabismus, mildly delayed motor development, or mildly abnormal speech. The remaining 11% suffered significant neurologic deficiencies including cerebral palsy, hemiparesis, and spastic quadriplegia. Of note, significantly more children in the neurologically impaired groups were born very preterm. Also of importance is that two of the most severely affected groups had abnormal brain scans before laser treatment. The findings of this study are consistent with those of Sutcliffe et al. who reported a cerebral palsy rate of 9% in children after in utero treatment with laser therapy for TTTS (93). In neurodevelopmental assessment following fetoscopic laser treatment of TTTS, impairment was observed in 12-18% of cases, half of which were severe (92,93). In multivariate analysis of cases of impairment, gestational age at birth was found to be the only significant factor (94).
In summary, a thoughtful approach to the management of TTTS requires consideration of every aspect of the presentation including gestational age, stage, Doppler findings, echocardiographic findings, concomitant placental insufficiency, and maternal risk factors. Until we have an effective medical therapy for TTTS, a judicious application of invasive procedures should be employed to optimize risk-benefit ratios for the mother and fetus.
Selective Fetoscopic Laser Photocoagulation for TTTS
Numerous treatments for TTTS have been proposed including selective feticide, cord coagulation, selective abdominal delivery (section parva), placental bloodletting, maternal digitalis, maternal indocin, serial amnioreduction, microseptostomy of the intertwin membrane, and nonselective or selective fetoscopic laser photocoagulation. For decades in the United States, serial amnioreduction had been the most widely accepted therapy for TTTS, but now selective fetoscopic laser photocoagulation is the standard of care with a much more limited role for amnioreduction and even less so for selective reduction.
The first treatment for TTTS that attempted to treat the anatomic basis for the syndrome was reported by DeLia et al. in 1990 (63), who described fetoscopic laser photocoagulatation of vessels crossing the intertwin membrane. At least in theory, this treatment option should be superior since it not only arrests shunting of blood from the donor to the recipient, but also halts the transfer of potential vasoactive mediators. In a subsequent series of patients, DeLia reported in 1995 a survival of 53% in 26 patients (64). While survival was not significantly better than previous reports with serial amnioreduction, the “neurologic outcome” in 96% of survivors was “normal” as assessed by head ultrasounds. Similarly, that same year Ville et al., reported 53% survival with a fetoscopic laser technique which was better than the 37% survival observed with historical controls at the same center with serial amnioreduction (65). They also observed a lower incidence of abnormalities detected by neonatal head ultrasound compared to historical controls.
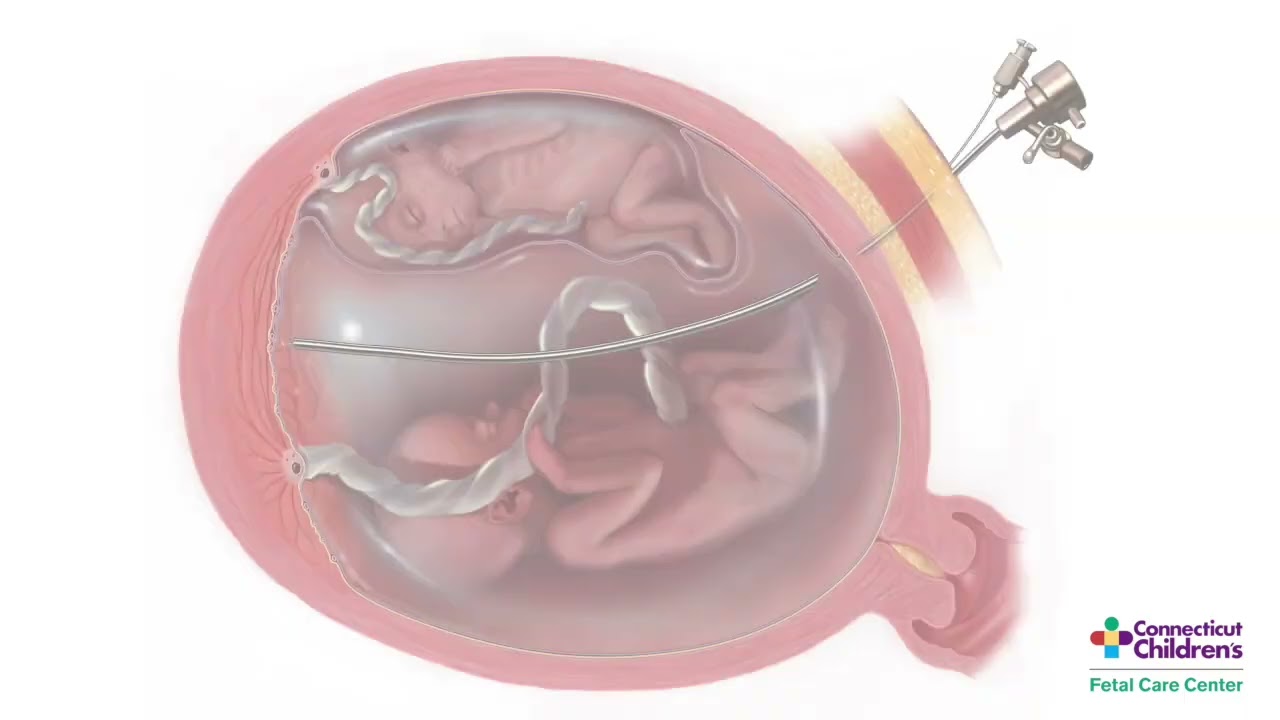
The non-selective fetoscopic laser technique photocoagulates all vessels crossing the intertwin membrane. This approach may be problematic, as the intertwin membrane often bears no relationship to the vascular equator of the placenta. This non-selective laser photocoagulation of all vessels crossing the intertwin membrane may sacrifice vessels not responsible for the TTTS, resulting in a higher death rate of the donor twin from acute placental insufficiency (66). A selective approach to fetoscopic laser photocoagulation in TTTS has been described by Quintero et al. (66). Unlike the non-selective coagulation technique initially described by DeLia, the selective technique does not photocoagulate every vessel crossing the intertwin membrane. Only direct, arterial-arterial and veno-venous connections are photocoagulated along with any unpaired artery going to a cotyledon with the corresponding vein (and vice versa) going to the opposite umbilical cord. Vessels on the chorionic plate can be differentiated endoscopically because arteries usually cross over veins and are darker in color due to lower oxygen saturation.
In a non-randomized comparison of patients treated by serial amnioreduction at one center and selective laser photocoagulation at another, the overall survival was not statistically significantly different (61% for laser vs. 51% for serial amnioreduction) (67). However, the survival of at least one twin with laser photocoagulation was 79%, while the survival of at least one twin with serial amnioreduction was only 60% (P < 0.05) (67).
The Eurofoetus trial for TTTS conducted by Senat et al. (68) was the first prospective randomized trial that compares the efficacy and safety of treatment of twin-twin transfusion syndrome with laser therapy versus serial amnioreduction. Women presenting between 15 and 26 weeks gestation with polyhydramnios in the recipient twin and oligohydramnios in the donor twin were allowed to participate. Fifty-two percent of patients were stage I or II, 47% were stage III and 1% were stage IV. Enrollment was halted after a planned interim analysis revealed a significantly higher likelihood of survival of at least one twin to 28 days of age (76% versus 56%, P=0.009) and to six months of age (76% versus 51%, p=0.002) in the laser group compared to the amnioreduction group. More infants were alive without neurologic abnormalities detected on neuroimaging studies in the laser group as well (52% versus 31%, P=0.003). The overall survival in the laser arm was 57%. This is consistent with previous reports of non-selective fetoscopic laser (53%) (63,64). This is significantly lower, however, than the survival reported with selective fetoscopic laser (64-68%) (69,70). Of particular concern is the poor survival which was observed in the amnioreduction arm. The overall survival was only 39%, which is significantly lower than previously reported (60-65%) (55,71). Antenatal, peripartum, and neonatal care were provided by the referring hospital and lack of standardization may explain some of these differences (72). The decreased survival in the amnioreduction group may reflect the higher pregnancy termination rate in the amnioreduction group (16 percent versus 0 percent in the laser group). The terminations were requested after the diagnosis of severe fetal complications; it would be instructive to know whether these women were offered cord coagulation as a means of rescuing one baby (72). A reliable assessment of neurologic outcome is critical when assessing the efficacy of treatment for TTTS. While there was a lower rate of abnormality on neurologic imaging in the laser group (7% versus 17%), there was no long-term neurodevelopmental assessment.
Quintero et al. retrospectively examined data from 78 patients treated by serial amnioreduction and 95 patients treated with selective laser photocoagulation with no significant difference in the distribution of patients by stage (69). Perinatal survival was not significantly different in the laser versus amnioreduction group (64.2% versus 57.7%). However, there was an inverse relationship between fetal survival and stage in the amniocentesis group but not in the laser group. For stage IV disease, there was significantly lower fetal survival in the amnioreduction group compared with the laser group (20.6% versus 63.6%, P=0.001). This information has important implications for the evaluation of treatment options and the development of stage-based treatment protocols.
Crombleholme et al, reported the result of the NIH-sponsored TTTS trial which differed significantly from the Eurofoetus trial reported by Senat in that the subjects prior to randomization had to have failed to respond to a single amnioreduction to exclude cases of the single amnioreduction paradox of an incidental septostomy (73). This study was stopped after 40 patients were randomized to fetoscopic laser versus serial amnioreduction when an increase in recipient mortality was noted in the fetoscopic laser arm. This was balanced by similar neonatal mortality in the amnioreduction arm. The two most important findings of this study were that the delay in definitive treatment can allow TTTS cardiomyopathy to progress and secondly, that there is a three-fold increase in recipient mortality with every point deduction in the Huhta Cardiovascular Profile Score used to define the severity of cardiac compromise (73).
Several European centers had observed a high incidence of post-operative twin anemia polycythemia sequence (TAPS) and persistent TTTS. Studies by Lapriore et al suggested that there were a number of communicating placental vessels evident on placental injection studies that had been missed at the time of the fetoscopic laser procedure (74). This led Oepkes and colleagues to suggest the Solomon technique as an adjunct to standard fetoscopic laser procedure in which after the communicating vessels had been identified and treated the intervening area of the placenta was photocoagulated to connect the areas of treatment and prevent small vascular connections from being missed (75). Slaghekke et al, reported the results of a prospective randomized study comparing the use of the Solomon technique to the standard selective fetoscopic laser technique (76). They found that in 274 women randomized the Solomon technique resulted in a significant reduction in post-operative TAPS from 16% to 3% and persistent TTTS from 7% to 1%. This approach has been adopted by many centers in Europe and the United States.
In our experience, the incidence of postoperative TAPS and persistent TTTS is << 1% with our current techniques. There are differences in the approach between the European centers and ours, which may account for this discrepancy. First, we use a fetoscope with a slightly larger lens, which may improve visualization of vessels, particularly small vessels, on the chorionic plate. In addition, we follow a mapping protocol that requires both operators to agree on the nature, size and location of all vascular connections. These are labeled and recorded intra-operatively during the first mapping. During the second mapping, each of the vascular connections is called out in sequence to be identified before being photocoagulated. Then a third mapping is performed to ensure that no vessel has been missed and there has been no recanalization which can occur particularly in large vessels. Since the incidence of complications due to the persistence of vascular connections (TAPS and TTTS) is already << 1%, we have not added the potential risk of laser photocoagulation to the placental area between vascular anastomoses to avoid increasing the risk of preterm premature rupture of membranes.
Volumes and Outcomes of Selective Fetoscopic Laser Photocoagulation for TTTS
Dr. Crombleholme has been a pioneer in the treatment of TTTS using selective fetoscopic laser photocoagulation for over 25 years and has performed over 1400 fetal surgical procedures making him one of the most experienced fetal surgeons in the world. His most recent outcomes among 246 patients with fetoscopic laser treatment for isolated TTTS are seen in Figure 1. The outcomes of fetoscopic laser treatment of TTTS are further stratified based on the presence of confounding variables as is the case when TTTS is complicated by selective fetal growth restriction (sFGR), presentation at < 18 weeks, or by a cervical length < 2 cm. Each of these conditions when seen in combination with TTTS, can adversely affect the outcomes as seen in Figure 2.
Overall Outcomes
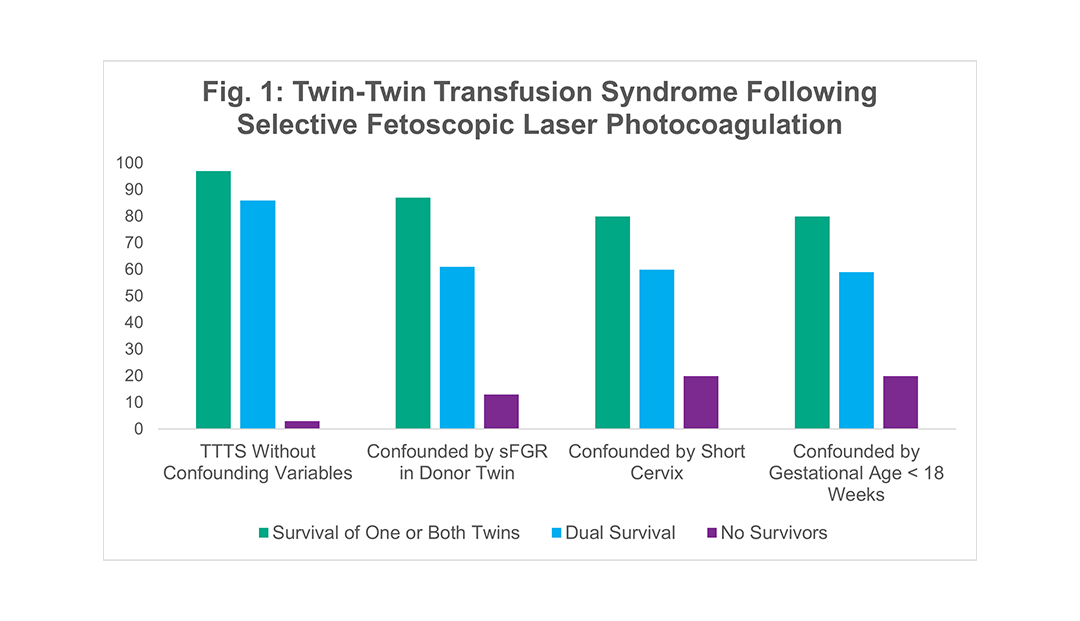
Figure 1 shows survival rates among 256 recent cases where Dr. Crombleholme used selective fetoscopic laser photocoagulation to treat TTTS. The best outcomes were in cases without confounding variables, but even cases with confounding variables had high survival rates.
Innovative Technique: Reduced Laser Time
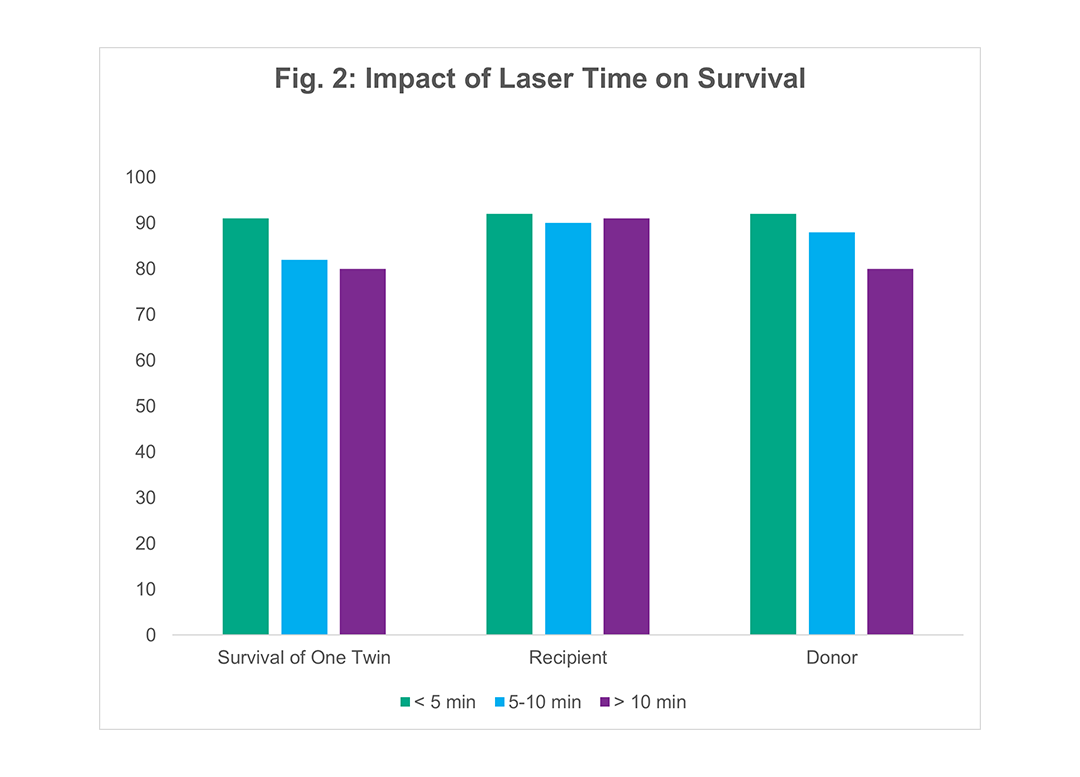
Figure 2 examines the effect of laser time on recipient and donor survival. Laser time, defined as the interval from laser photocoagulation of the first vessel to the last vessel, was determined for all cases of TTTS treated by fetoscopic laser photocoagulation. The survival of the recipient twins was unaffected by laser time. Donor survival, however improved as the duration of laser time was reduced. The longer laser time also correlated with number of vascular connections (number of vessels). There was also a correlation of increased laser time with increased peak MCA Doppler flow velocity in the donor twin post-operatively indicating anemia in the donor twin. This suggests that a true transfusion between donor and recipient occurs during prolonged laser times.
Innovative Technique: Nifedipine
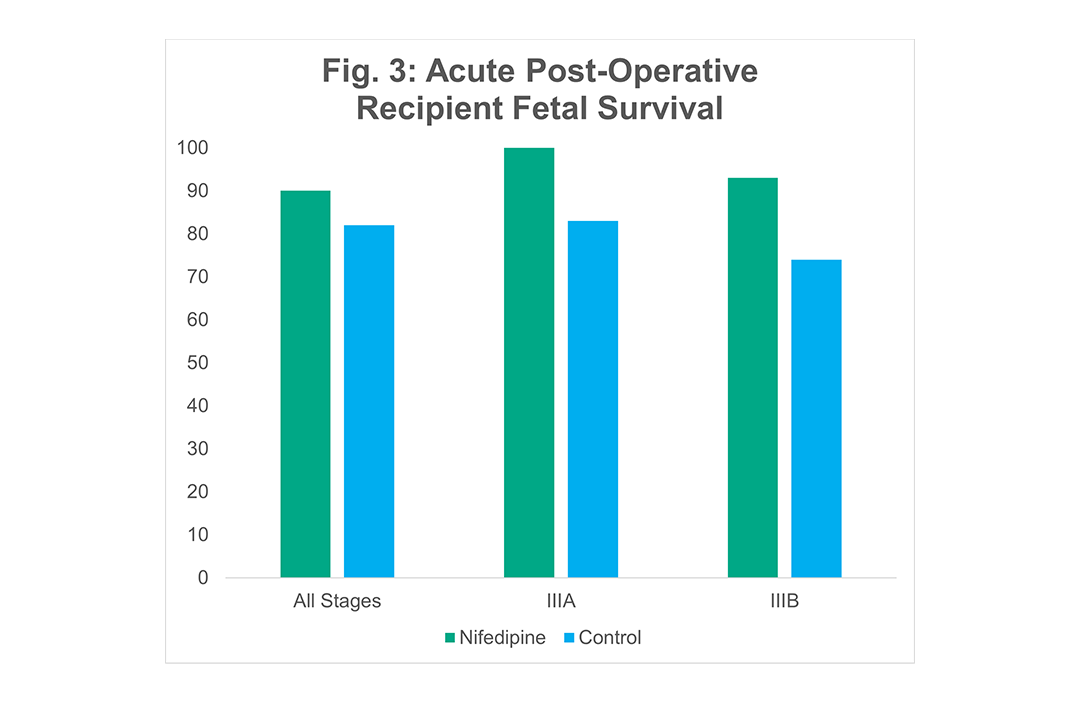
Nifedipine has been shown to improve survival of the recipient twin but has no effect on the donor twin. In a prospective cohort study of 141 pregnant mothers with TTTS treated with nifedipine (20 mg q 6 hours), there was a statistically significant improvement in recipient survival when compared to gestational age and stage matched controls.
Other Treatment Options
Amnioreduction was first employed for maternal comfort and as a means to control polyhydramnios in the hope of prolonging the pregnancy until the risks of extreme prematurity are lessened. In addition, amnioreduction improves uteroplacental blood flow, likely by reduction of pressure from the amniotic fluid. In uncontrolled series, amnioreduction improves survival compared to the natural history of untreated TTTS. Moise, in a review of 26 reports dating from the 1930s of 252 fetuses, found an overall survival of 49% (77). The survival in more recent series, with more consistently aggressive serial amnioreduction to reduce amniotic fluid volume to normal, have ranged widely from as low as 37% to as high as 83% (78-83). However, these retrospective series are comprised of small numbers of patients from a range of gestational ages, as well as from a spectrum of severity of TTTS. The severity of TTTS and gestational age at diagnosis may have a profound impact on the observed mortality with any treatment strategy. The earlier in gestation TTTS presents, the worse the prognosis. Mari et al. found that patients presenting with advanced TTTS prior to 22 weeks gestation and absent end diastolic flow in the recipient umbilical artery had a survival of both twins with aggressive amnioreduction of only 13% and, with absent end diastolic flow in the donor umbilical artery, survival was 33% (55).
Some centers in the United States and Europe advise against ever treating TTTS by amnioreduction because of the risk of chorioamniotic separation which they consider a contraindication to fetoscopic laser surgery. The risk of chorioamniotic separation is between 1 and 3% in our hands, and even when chorioamniotic separation occurs, we have been able to perform fetoscopic laser surgery. In fact, we have treated several cases of global chorioamniotic separation by an extra-amniotic fetoscopic laser technique using hydrodissection. The outcomes in these cases have mirrored our outcomes with TTTS without chorioamniotic separation albeit with a mean gestational age at delivery 1-2 weeks earlier.
The paradoxical resolution of oligohydramnios after a single amnioreduction was first suggested by Saade et al. to be due to inadvertent puncture of the intertwin membrane (84). Intertwin septostomy was specifically proposed as a treatment for TTTS to restore amniotic fluid dynamics without the need for repeated amnioreduction. One objection to this approach is the possibility it would result in a large septostomy, creating an essentially monoamniotic sac with the attendant risk of cord entanglement. For this reason, a fetoscopic “microseptostomy” has been proposed as an adjunct to fetoscopic laser surgery with a 600-micron diameter to prevent this complication (see below). In a small multicenter series of 12 patients, Saade et al., reported an 81% survival with microseptostomy (85).
However, not only was this series small and uncontrolled, there was no report of neurologic or cardiac morbidity. In a direct comparison, albeit a small retrospective single-institution series, of serial amnioreduction versus microseptostomy, Johnson et al. observed no survival advantage with either therapy (71). This was confirmed by Saade et al. who reported the results of a multicenter prospective randomized clinical trial comparing amnioreduction to septostomy. The survival in each arm of the study was 65% (86) consistent with the notion that the effect of amnioreduction may be inadvertent septostomy. These studies, however, cannot prove this theory. Ultrasound-guided septostomy with a needle has largely been abandoned due to the risk of creating a monoamniotic sac and cord entanglement putting both fetuses at risk. Dual fetal demise as a result of a cord accident arising from cord entanglement has been reported following needle septostomy.
Echocardiographic data in TTTS shows that the recipient twin has significant hypertension, which results in progressive cardiomegaly, myocardial hypertrophy, and diastolic dysfunction. After the results of the NIH trial showed a three-fold increased mortality in recipients for each point deduction in the Huhta CVPS score, we reasoned that if we treated fetal hypertension with an adjunctive medical therapy to fetoscopic laser surgery, we may improve the recipient survival. We empirically began treating mothers of twins with TTTS with nifedipine for 24 to 48 hours prior to fetoscopic surgery and compared outcomes to mothers who were not treated with nifedipine. Nifedipine is the most commonly used anti-hypertensive agent used during pregnancy, crosses the placenta, and achieves similar levels in the fetus as the mother. We treated 141 cases of TTTS with nifedipine and compared them to 152 gestational age and stage-matched controls (87). We observed a statistically significant improvement in overall fetal and neonatal survival with 90% survival with nifedipine and 82% (p < 0.017) without nifedipine. There was no effect on donor survival and the survival benefit was limited to the recipient twins. In stage IIIA, recipient survival was 100% with nifedipine versus 83% without, and in IIIB, it was 93% with versus 74% without. This survival benefit was observed not just in acute post-operative survival but also in neonatal survival.
The improved recipient survival with maternal treatment of nifedipine was evident in the immediate post-operative period and there was no significant change in survival to birth suggesting the survival benefit was during the perioperative period and was not related to tocolytic effects of nifedipine. We looked at the levels of brain-atrial natriuretic peptide (BNP) in the amniotic fluid of control twins, versus TTTS patients treated with nifedipine versus TTTS patients not treated with nifedipine and found a significant reduction in BNP levels suggesting a reduction in myocardial strain in patients treated with nifedipine which may have contributed to the improved perioperative survival (88).
Some centers have taken the view that the most definitive approach to treating TTTS is selective reduction using fetoscopic cord ligation or coagulation. The rationale for this approach is that cord occlusion and sacrifice of one twin arrests the syndrome, prolongs the gestation, and maximizes the outcome for one twin. We have reserved this approach for instances where advanced TTTS cardiomyopathy has irretrievably compromised the recipient twin with no hope for salvage. In such cases, due to unequal sharing between the donor and recipient, the selective fetoscopic laser procedure may result in the death of the donor twin from acute placental insufficiency within hours of the procedure and a recipient twin that dies from progressive TTTS cardiomyopathy. In this situation, fetoscopic cord coagulation may be the best option available. Cord coagulation preserves the vascular communications between the donor twin and the recipient twin’s placenetal domains. In 16 of 17 such cases, we have observed rebound fetal growth, restoration of amniotic fluid volume, and delivery of neurologically intact donor twins at a mean gestational age of 34 weeks. One survivor had grade I intraventricular hemorrhage but is otherwise doing well.
The most common clinical presentation in which selective reduction is considered in the treatment of TTTS is in stage IV when the recipient twin develops hydrops. Many patients may be unwilling to consider selective reduction because of personal, ethical, or religious reasons. In these cases, selective fetoscopic laser photocoagulation may be a better option as it separates the fate of the twins but without sacrificing one or the other twin. We reviewed our experience with the management of stage IV TTTS. Among these 50 cases, 2 declined treatment, 13 opted for selective reduction by radiofrequency ablation (RFA) and 35 underwent fetoscopic laser procedure. As would be expected, there was a 50% survival in those cases being treated by selective reduction by RFA. Surprisingly, the overall survival with fetoscopic laser in these stage IV cases was 90% with 100% of cases having at least one survivor and 80% of cases having dual survivors. The donor survival was 100% and the recipient survival was 80% with resolution of hydrops and improvement in cardiac function. Further analysis showed that in stage IV TTTS if the recipient twin had pulmonic atresia combined with severe LV dysfunction, the mortality is 80%. In contrast, if there is no pulmonary atresia or no LV dysfunction, the recipient's survival is 100%. In cases in which there was either pulmonic atresia or severe LV dysfunction, the recipient survival with fetoscopic laser was 88%. Due to these results, we rarely offer a selective reduction for stage IV TTTS, unless there is evidence on fetal MRI of brain injury or at the specific request of the parents.
There is a broad range of severity in TTTS at presentation and not all cases warrant going immediately to fetoscopic laser surgery. In cases of TTTS manifested only by amniotic fluid discordance meeting criteria for Quintero stage I, there is at least a 30-50% chance of the TTTS resolving spontaneously without any intervention (89). Because this propensity to spontaneously improve is limited to cases without evidence of TTTS cardiomyopathy, we use fetal echocardiography to select patients with early TTTS for observation or amnioreduction as initial management. In cases in which the recipient DVP is < 10 cm, we tend to favor observation, but in cases in which the DVP is >10, we suggest a trial of a single amnioreduction.
In a series of 585 cases, 212 were found to have no or minimal fetal echocardiographic abnormalities (i.e., Cincinnati stage I, II, III, and IIIA). Of the 212, 158 had either observation alone or a trial of a single amnioreduction with ultrasound and fetal echocardiographic surveillance for signs of TTTS progression. The remaining 54 cases went directly to selective fetoscopic laser photocoagulation. Among those managed by observation alone 81% (38/47) spontaneously improved and did not require any invasive procedure. Among the 111 who underwent a trial of a single amnioreduction, 55% responded with normalization of amniotic fluid volumes and arrest of TTTS. Those who progressed either by worsening polyhydramnios, development of Doppler changes, or echocardiographic signs of TTTS cardiomyopathy in the observation group (19%, 9/47) or the single amnioreduction group (45%, 51/111) went on to have fetoscopic laser surgery. Progression was detected by fetal echocardiogram alone in 45%, ultrasound alone in 32%, and in both studies in 23%.
Survival in each of these groups was found to be the same. Those who spontaneously improved during observation only had an overall survival of 87% (66/76), while those who had a single amnioreduction and responded had a survival of 86% (103/120). Those cases that went directly to fetoscopic laser surgery had an overall survival rate of 81% (87/108). Those patients who initially were managed either by observation or a single amnioreduction but showed signs of progression of TTTS and subsequently underwent fetoscopic laser photocoagulation, had an overall survival of 79% (95/120), which was not statistically significantly different from any of the other groups. The importance of these results is that there are no adverse consequences from either an initial period of observation or a trial of response to a single amnioreduction. By this approach, we avoided the potential risks of more invasive procedures in 62% of cases with TTTS without cardiomyopathy.
Among those who underwent amnioreduction, 9 developed chorioamniotic separation, and all but 2 underwent subsequent fetoscopic laser surgery. At the time of this series, we were not performing fetoscopic laser surgery in cases of global chorioamniotic separation, but now we are.
1. Sueters M, Middeldorp JM, Lopriore E, et al. Timely diagnosis of twin-to-twin transfusion syndrome in monochorionic twin pregnancies by biweekly sonography combined with patient instruction to report onset of symptoms. Ultrasound Obs Gynecol 2006;28(5):659-664.
2. Couck I, Ponnet S, Thewissen L, et al. The Detection, Outcome, and Presentation of Twin-Twin Transfusion Syndrome in Monochorionic Diamniotic Twin Pregnancies Followed with a Protocol of Fortnightly Ultrasound Examination. Fetal Diagn Ther April 2021:1-8.
3. Quintero RA, Morales WJ, Allen MH et al. Staging of twin-twin transfusion syndrome. J Perinatol 1999; 19: 550-5.
4. Taylor MJO, Govender L, Jolly M et al. Validation of the Quintero staging system for twin-twin transfusion syndrome. Obstet Gynecol 2002; 100:1257-1265.
5. Van Mieghem T, De Heus R, Lewi L, et al. Prenatal management of monoamniotic twin pregnancies. Obstet Gynecol 2014;124(3):498-506.
6. Barrea C, Alkazaleh F, Ryan et al. Prenatal cardiovascular manifestations in the twin-to-twin transfusion syndrome recipients and the impact of therapeutic amnioreduction. Am J Obstet
7. Tan TYT, Taylor MJO, Lee LY et al. Doppler for artery-artery anastomosis and stage-independent survival in twin-twin transfusion. Obstet Gynecol 2004; 103: 1174-80.
8 Michelfelder E et al. Abstract, American Academy of Pediatrics Annual Meeting 2005.
9. Fisk NM, Borrell A, Hubinont C et al. Fetofetal transfusion syndrome: Do the neonatal criteria apply in utero? Arch Dis Child 1990; 65: 657-61.
10. Wee LY, Taylor M, Watkins N et al. Characterisation of deep arterio-venous anastomoses within monochorionic placentae by vascular casting. Placenta 2005; 26:19-24.
11. Mahieu-Caputo D, Dommergues M, Delezoide AL et al. Twin-to-twin transfusion syndrome: role of the fetal renin-angiotensin system. Am J Pathol 2000; 156: 629-36.
12. Duncombe GJ, Dickinson JE, Evans SF. Perinatal characteristics and outcomes of pregnancies complicated by twin-twin transfusion syndrome. Obstet Gynecol 2003; 101: 1190-
13. Senat M V, Deprest J, Boulvain M, et al. Endoscopic Laser Surgery versus Serial Amnioreduction for Severe Twin-to-Twin Transfusion Syndrome. N Engl J Med 2004;351(2):136-144.
14. Ichizuka K, Matsuoka R, Hasegawa J et al. The Tei index for evaluation of fetal myocardial performance in sick fetuses. Early Hum Dev 2005; 81:273-9.
15. Rychik J , Tian Z, Bebbington M, Xu F, McCann M, Mann S, Wilson RD, Johnson MP: The twin-twin transfusion syndrome: Spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol 197: 392e1-8, 2007
16. Quintero RA, Morales WJ, Allen MH, et al. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19(8 Pt 1):550-555.
17. Ville Y. Twin-to-twin transfusion syndrome: Time to forget the Quintero staging system? Ultrasound Obstet Gynecol 2007;30(7):924-927.
18. Bamberg C, Diehl W, Diemert A, et al. Neither the differentiation between twin-twin transfusion syndrome Stages I and II nor III and IV makes a difference regarding the probability of double survival after laser therapy. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol September 2020.
19. Rychik J. Fetal Cardiovascular Physiology. Pediatr Cardiol 2004; 25: 201-9.
20. Gapp-Born E, Sananes N, Guerra F, Kohler M, Weingartner AS, Fritz G, Viville B, Langer B, Sauleau E, Nisand I, Farve R. Predictive value of cardiovascular parameters in stages 1 and 2 of twin-to-twin transfusion syndrome Prenatal Diag 2014; 34:908-914
21. Gratacos E, Lewi L, Munoz B, Acosta-Rojas R, Hernandez-Andrade E, Martinez JM, Carreras E, Deprest J: A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol 2007; 30: 28-34
22. Sebire N, Snijders R, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol 1997; 104: 1203–1207.
23. Quintero RA, Bornick PW, Morales WJ, Allen MH. Selective photocoagulation of communicating vessels in the treatment of monochorionic twins with selective growth retardation. Am J Obstet Gynecol 2001; 185: 689–696.
24. Gratacos E, Carreras E, Becker J, Lewi L, Enriquez G, Perapoch J, Higueras T, Cabero L, Deprest J. Prevalence of neurological damage in monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end-diastolic umbilical artery flow. Ultrasound Obstet Gynecol 2004; 24: 159–163.
25. Chauhan SP, Shields D, Parker D, Sanderson M, Scardo JA, Magann EF. Detecting fetal growth restriction or discordant growth in twin gestations stratified by placental chorionicity. J Reprod Med 2004; 49: 279–284.
26. Vanderheyden TM, Fichera A, Pasquini L, Tan TY, Wee LY, Frusca T, Fisk NM. Increased latency of absent end-diastolic flow in the umbilical artery of monochorionic twin fetuses. Ultrasound Obstet Gynecol 2005; 26: 44–49.
27. Victoria A, Mora G, Arias F. Perinatal outcome, placental pathology, and severity of discordance in monochorionicand dichorionic twins. Obstet Gynecol 2001; 97: 310–315.
28. Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol 2004; 190: 156–163.
29. Adegbite AL, Castille S, Ward S, Bajoria R. Prevalence of cranial scan abnormalities in preterm twins in relation to chorionicity and discordant birth weight. Eur J Obstet Gynecol Reprod Biol 2005; 119: 47–55.
30. Huber A, Diehl W, Zikulnig L, Bregenzer T, Hackeloer BJ, Hecher K. Perinatal outcome in monochorionic twin pregnancies complicated by amniotic fluid discordance without severe twin–twin transfusion syndrome. Ultrasound Obstet Gynecol 2006; 27: 48–52.
31. Hecher K, Jauniaux E, Campbell S, Deane C, Nicolaides K. Artery-to-artery anastomosis in monochorionic twins. Am J Obstet Gynecol 1994; 171: 570–572.
32. Gratacos E, Lewi L, Carreras E, Becker J, Higueras T, Deprest J, Cabero L. Incidence and characteristics of umbilical artery intermittent absent and/or reversed end-diastolic flow in complicated and uncomplicated monochorionic twin pregnancies. Ultrasound Obstet Gynecol 2004; 23: 456–460.
33. Slaghekke F, Kist WJ, Oepkes D, et al. Twin anemia-polycythemia sequence: Diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther. 2010;27(4):181-190. doi:10.1159/000304512
34. Robyr R, Lewi L, Salomon LJ, et al. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2006;194(3):796-803. doi:10.1016/j.ajog.2005.08.069
35. Slaghekke F, van Klink JMM, Koopman HM, Middeldorp JM, Oepkes D, Lopriore E. Neurodevelopmental outcome in twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2014;44(3):316-321. doi:10.1002/uog.13387
37. Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199(5):514.e1-8. doi:10.1016/j.ajog.2008.03.050
38. Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47(2):247-263. doi:10.1002/uog.15821
39. Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Prevalence of placental dichotomy, fetal cardiomegaly and starry‐sky liver in twin anemia polycythemia sequence. Ultrasound Obstet Gynecol. December 2019. doi:10.1002/uog.21948
40. Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Improved prediction of twin anemia–polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound Obstet Gynecol. 2019;53(6):788-793. doi:10.1002/uog.20096
41. Khalil A, Beune I, Hecher K, et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol. 2019;53(1):47-54. doi:10.1002/uog.19013
42. Tollenaar LSA, Slaghekke F, Lewi L, et al. Spontaneous Twin Anemia Polycythemia Sequence: Diagnosis, Management and Outcome in an International Cohort of 249 Cases. Am J Obstet Gynecol. July 2020. doi:10.1016/j.ajog.2020.07.041
43. Tollenaar LSA, Lopriore E, Faiola S, et al. Post-Laser Twin Anemia Polycythemia Sequence: Diagnosis, Management, and Outcome in an International Cohort of 164 Cases. J Clin Med. 2020;9(6):1759. doi:10.3390/jcm9061759
44. Tollenaar LSA, Slaghekke F, Lewi L, et al. Treatment and outcome in 370 cases with spontaneous or post‐laser twin anemia polycythemia sequence managed in 17 different fetal therapy centers. Ultrasound Obstet Gynecol. April 2020. doi:10.1002/uog.22042
45. Bajoria R, Sullivan M, Fisk NM. Endothelin concentrations in monochorionic twins with severe twin-twin transfusion syndrome. Human Reprod 1999; 14 (6): 1614-18.
46. Bajoria R, Ward S, Chatterjee R. Brain natriuretic peptide and endothelin-1 in the pathogenesis of polyhydraminos-oligohydraminos in monochorionic twins. Am J Obstet Gynecol 2003; 189: 189-94.
47. Yang SG, Tian ZY, Cohen MS et al. Fetal echocardiographic findings in twin-twin transfusion syndrome. J Am Soc Echocardiogr 2000; 13: 452 (abstr).
48. Lougheed J, Sinclair BG, Fung KFK et al. Acquired right ventricular outflow tract obstruction in the recipient twin in twin-twin transfusion syndrome. J Am Coll Cardiol 2001; 38: 1533-8.
49. Habli M, Cromblehole TM, Livingston J, Herman J, Lim FY, Michelfelder E: Acute effects of fetoscopic laser photocoagulation on recipient cardiac function in twin-twin transfusion syndrome. Am J Obstet Gynecol 199(4): 412 e1-6, 2008
50. Manning N, Archer N: Cardiac manifestations of twin-twin transfusion syndrome. Twin Res Genet 19(3)246-254, 2016
51. Michelfelder E, Tan X, Cnota J, Divanovic A, Statile C, Lim FY, Crombleholme TM: Prevalence, spectrum, and outcome of right ventricular outflow tract abnormalities in twin-twin transfusion syndrome: A large single-center experience. Congenit Heart Dis10(3): 209-218, 2015
52. van den Boom J, Battin M, Hornung T: Twin-twin transfusion syndrome, coarctation of the aorta, and hypoplastic aortic arch: A case series report. J Paediatr Child Health 46(3): 78-79, 2010
53. Puetz JD, Schrager SM, Wang TV, Llanes A, Chmait RH, Venderbilt DL: Blood pressure evaluation of children treated with laser surgery for twin-twin transfusion syndrome at two years follow up. Am J Obstet Gynecol 213(3): 417e1-e7, 2015
54. Gardiner HM, Matsul H, Roughton M, Greenwald SF, Diemert A, Taylor MJO, Hecher K: Cardiac function in 10-year-old twins following different fetal therapies for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 43(6): 652-657, 2014
55. Mari G, Roberts A, Detti L al. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome: Results of the International Amnioreduction Registry. Am J Obstet Gynecol 2001; 185: 708-15.
56. Martinez JM, Bermudez C, Becerra C et al. The role of Doppler studies in predicting individual intrauterine fetal demise after laser therapy for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2003; 22: 246-51.
57. Zikulnig L, Hecher K, Bregenzer T et al. Prognostic factors in severe twin-twin transfusion syndrome treated by endoscopic laser surgery. Ultrasound Obstet Gynecol 1999; 14: 380-87.
58. Ville Y, Hecher K, Gagnon A. Endoscopic laser coagulation in the management of severe twin-to-twin transfusion syndrome. Br J Obstet Gynecol 1998; 105: 446-53.
59. Karatza AA, Wolfenden JL, Taylor MJO et al. Influence of twin-twin transfusion syndrome on fetal cardiovascular structure and function: prospective case-control study of 136 monochorionic twin pregnancies. Heart 2002; 88: 271-7.
60. Simpson LL, Marx GR, Elkadry EA et al. Cardiac dysfunction in twin-twin transfusion syndrome: a prospective, longitudinal study. Obstet Gynecol 1998; 92: 557-62.
61. Fesslova V, Villa L, Nava S et al. Fetal and neonatal echocardiographic findings in twin-twin transfusion syndrome. Am J Obstet Gynecol 1998; 179: 1056-62.
62. Gardiner HM, Taylor MJO, Karatza A et al. Twin-twin transfusion syndrome: the influence of intrauterine laser photocoagulation on arterial distensibility in childhood. Circulation 2003; 107: 1906-11.
63. De Lia JE, Cruikshank DP, Kaye WR. Fetoscopic neodymium: YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol 1990; 75: 1046-1053.
64. De Lia JE, Kuhlmann RS, Harstad TW et al. Fetoscopic laser ablation of placental vessels in severe twin-twin transfusion syndrome. Am J Obstet Gynecol 1995; 172: 1202-1211.
65. Ville Y, Hyett J, Hecher K, Nicolaides KH. Preliminary experience with endoscopic laser surgery for severe twin-twin transfusion syndrome. N Engl J Med 1995; 332: 224-227.
66. Quintero RA, Morales WJ, Mendoza G, et al. Selective photocoagulation of placental vessels in twin-twin transfusion syndrome: Evolution of a surgical technique. Obstst Gynecol Survey 1998; 53: 597-603.
67. Hecher K, Plath H, Bregenzer T, et al. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol 1999; 180: 717-24.
68. Senat MV, Deprest J, Boulvain M et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004; 351: 136-44.
69. Quintero RA, Dickinson JE, Morales WJ et al. Stage-based treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol 2003; 188: 1333-40.
70. Hecher K, Diehk W, Zikulnig L et al. Endoscopic laser coagulation of placental anastomoses in 200 pregnancies with severe mid-trimester twin-to-twin transfusion syndrome. Eur J Obstet Gynecol Reprod Biol 2000; 92: 135-9.
71. Johnson JR, Rossi KQ, Shaughnessy RW et al. Amnioreduction versus septostomy in twin-twin transfusion syndrome. Am J Obstet Gynecol 2001; 185: 1044-1047.
72. Fisk NM, Galea P. Twin-twin transfusion – As good as it gets? N Engl J Med 2004; 351: 182-4.
73. Crombleholme TM, Shera D, Lee H et al: a prospective randomized multicenter trial of amnioreduction versus selective fetoscopic laser photocoagulation for the treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol 197(4): 396 e1-e9, 2007
74. Lopriore E, Slaghekke F, Middeldorp JM, Klumper FJ, vonLith JM, Walther FJ, Oepke D: Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. J Vis Exp 55: 3208, 2011
75. Slaghekke F, Oepkes D: Solomon technique versus selective coagulation for twin-twin transfusion syndrome. Twin Res Hum Genet 19(3): 217-221, 2016
76. Slaghekke F, Lopriore E, Lewi L, et al: Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-twin transfusion syndrome: An open label randomized controlled trial. Lancet 383: 2144-2151, 2014
77. Moise KJ Jr. Polyhydramnios: problems and treatment. Semin Perinatol 1993; 17: 197-209.
78. Rodestal A, Thomassen PA. Acute polyhydramnios in twin pregnancy. A retrospective study with special reference to therapeutic amniocentesis. Acta Obstetricia et Gynecologica Scandinavic 1990; 69: 297-300.
79. Urig MA, Clewell WH, Elliott J. Twin-twin transfusion syndrome. Am J Obstet Gynecol 1990; 163: 1522-1526.
80. Mahony BS, Petty CN, Nyberg DA et al. The “stuck twin” phenomenon: ultrasonographic findings, pregnancy outcome, and management with serial amniocenteses. Am J Obstet Gynecol 1990; 163: 1513-1521.
81. Elliott JP, Urig MA, Clewell WH. Aggressive therapeutic amniocentesis for treatment of twin-twin transfusion syndrome. Obstet Gynecol 1991; 77: 537-540.
82. Pinette MG, Pan Y, Pinette SG et al. Treatment of twin-twin transfusion syndrome. Obstet Gynecol 1993; 82: 841-846.
83. Reisner DP, Mahony BS, Petty CH et al. Stuck twin syndrome: outcome in 37 consecutive cases. Am J Obstet Gynecol 1993; 169: 991-995.
84. Saade GR, Olson G, Belfort MA et al. Amniotomy: a new approach to the “stuck twin” syndrome. Am J Obstet Gynecol 1995; 172: 429-434.
85. Saade GR, Belfort MA, Berry DL et al. Amniotic septostomy for the treatment of twin oligohydramnios-polyhydramnios sequence. Fetal Diagn Ther 1998; 13: 86-93.
86. Saade G, Moise K, Dorman K et al. A randomized trial of septostomy versus amnioreduction in the treatment of twin oligohydramnios polyhydramnios sequence (TOPS). Am J Obstet Gynecol (Society for Maternal -Fetal Medicine. Oral presentation abstract 3): 187 (6), 2003.
87. Crombleholme TM, Lim FY, Habli M et al: Improved recipient survival with maternal nifedipine in twin-twin transfusion syndrome complicated by TTTS cardiomyopathy undergoing selective fetoscopic laser photocoagulation. Am J Obstet Gynecol 203(4): 397e1-e9, 2010
88. Habli M, Cnota, Michelfelder E, Salisbury S, Schnell B, Polzin W, Lim FY, Crombleholme TM: The relationship between amniotic fluid levels of brain-type naturietic peptide and recipient cardiomyopathy in twin-twin transfusion syndrome. Am J Obstet Gynecol 203 (4) 404 e1-7, 2010
89. Washburn EE, Sparks TN, Gosnell KA, et al. Stage I Twin-Twin Transfusion Syndrome: Outcomes of Expectant Management and Prognostic Features. Am J Perinatol 2018;35(14):1352-1357.
90. Quintero RA, Martinez JM, Bermudez et al. Fetoscopic demonstration of perimortem feto-fetal hemorrhage in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2002; 20: 638-9.
91. Lopriore E, Nagel H, Vandenbussche F et al. Long-term neurodevelopmental outcome in twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2003; 189: 1314-9.
92. Lenclen R, Ciarlo G, Paupe A, et al. Neurodevelopmental outcome at 2 years in children born preterm treated by amnioreduction or fetoscopic laser surgery for twin-to-twin transfusion syndrome: comparison with dichorionic twins. Am J Obstet Gynecol 2009;201(3):291.e1-5.
93. Lopriore E, Ortibus E, Acosta-Rojas R, et al. Risk factors for neurodevelopment impairment in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol 2009;113(2 Pt 1):361-366.
94. Brandsma FL, Spruijt MS, Rijken M, et al. Behavioural outcome in twin-twin transfusion syndrome survivors treated with laser surgery. Arch Dis Child Fetal Neonatal Ed 2020;105(3):304-309.