Fetal alloimmunization, previously referred to as isoimmunization, occurs when a pregnant person’s immune system produces antibodies against fetal red blood cell antigens which can attack and destroy the fetal red blood cells (hemolytic anemia).
Background
Every individual has red blood cells that are responsible for transporting oxygen from the lungs to the tissues of the body. Red blood cells have multiple different types of inherited antigens, which are proteins or carbohydrates present on the red blood cell surface. These antigens define our blood type and immune response. Most commonly we understand these as ABO antigens. For example, people with type A blood have A antigens, type B have B antigens, Type O blood has neither, and AB has both. Some red blood cell antigens are well known (for example Rhesus antigens which can lead to Rh disease due to the presence of Rh D antigen), and other lesser known but clinically important antigens (Kell, Kidd, and Duffy). There are over 300 known red blood cell antigens.
Pregnant patients can become sensitized to foreign antigens through exposure to blood transfusions, pregnancy and childbirth, and any other form of foreign blood component contamination.
Identification of a maternal antibody response to fetal red cell antigens and follow-up screening allows for treatment, and sometimes even prevention, of fetal alloimmunization in pregnancy. Depending on the antigen type, antibody formation and transplacental passage, there can be significant risks to the fetus leading to hemolytic anemia (when the attacking immune system causes the breakdown of fetal red blood cells), heart failure and stillbirth. Most commonly this is seen in Rh Disease, which can cause devastating anemia in the fetus. The introduction of Rhogam in the late 1960s has dramatically reduced the incidence of fetal death among patients with Rh Disease to 1-2%.
At the Fetal Care Center at Connecticut Children’s, we screen for and manage cases of alloimmunization in order to preserve the life of the affected fetus. Below are some common questions patients may have as they navigate a pregnancy complicated by alloimmunization.
As noted above, alloimmunization can lead to hemolytic disease of the fetus/newborn (HDFN). This is a rare disease where the fetus develops severe anemia secondary to the hemolysis or destruction of fetal red blood cells due to maternal red cell antibody/fetal red cell antigen incompatibility. This can lead to fetal hydrops due to cardiomyopathy and heart failure resulting from severe fetal anemia. Fetal hydrops presents with fluid collections in multiple fetal compartments including the skin, abdominal cavity, lungs, pericardium (fluid filled sac that surrounds and protects the heart) and even the amniotic fluid. Ultimately, this can lead to fetal death. In some cases, the mother can also develop similar symptoms including swelling or edema and elevated blood pressures. This is known as mirror syndrome and while quite rare, has high maternal morbidity.
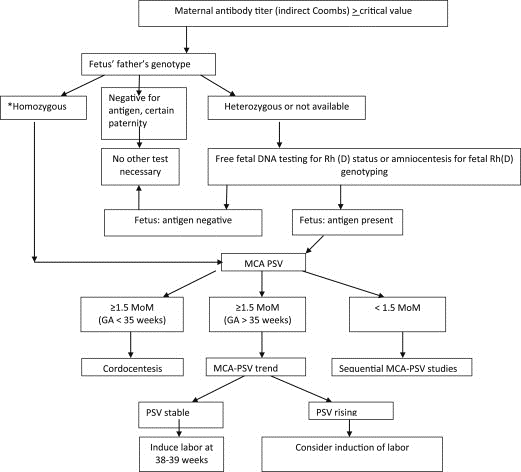
At the initial prenatal visit, pregnant patients undergo a serum screen (blood test) to determine maternal blood type and presence of red blood cell antibodies. These antibodies may have formed due to maternal exposure to foreign red blood cell antigens, such as from prior pregnancies or from a history of blood transfusion. If identified, the next step is to test paternal blood to determine whether antigens to the antibody are present and therefore have a chance of being inherited by the fetus. This is either done through DNA analysis or serologies/paternal blood testing.
If there is the possibility for fetal inheritance of a paternal antigen (e.g. if the father is either homozygous or heterozygous for that antigen), various methodologies can be used to determine next steps. For instance, new genetic screening technology is now available through certain diagnostic labs, such as with Natera, that allows identification of the Rh status of the fetus without performing invasive diagnostic testing. Currently, in other countries, some of the minor Rhesus antigens can also be identified. Conversely, invasive diagnostic testing through amniocentesis can be performed for fetal testing in instances when non-invasive testing is not available. Amniocentesis can carry risks, however, with approximately a 1:900 risk of loss, so many parents opt for screening with maternal antibody titers in the event there is a chance the fetus may carry the same antigen as the father.
If the paternal testing is negative for a particular antigen, and paternity is assured, no further testing is necessary.
In the majority of cases where there is a known maternal antibody and a concern for discordant paternal antigen, titers can be followed. This is usually performed monthly in early pregnancy with the interval shortened in the case of changes or concerns. Antibody titers measure the concentration of an antibody in the maternal serum—it is done with serial dilutions of 1:2 until no further antibody can be detected. For instance, at a titer of 1:8, antibody was still detected after dilution of 1 part serum to 8 parts of a solution. Higher antibody titers correlate to a stronger antibody response with more antibodies present (this in turn increases the likelihood of transplacental passage and resultant hemolysis and fetal anemia). A critical titer in most institutions and for most antibodies is usually between 1:16 to 1:32. When this critical titer is reached, middle cerebral artery (MCA) Doppler assessment is initiated. (This is generally not initiated until after 16 weeks of gestation).
MCA Dopplers rely on measurement of the peak systolic velocity (MCA-PSV), or speed the blood travels through a vessel in the brain, to detect fetal anemia. Velocities are gestational-age dependent and a value greater than 1.5 MoM or multiples of the median correlates to increased risk for fetal anemia. It is important to have MCA-PSV completed in a center well trained in obtaining these measurements, such as with the Fetal Care Center at Connecticut Children’s, because they are highly dependent on the ultrasound angle of insonation, and can be affected by fetal movement and heart rate accelerations (Crombleholme). They can also be affected by later gestational ages, and earlier gestational ages require specific algorithms (See Figure 1).
Figure 1
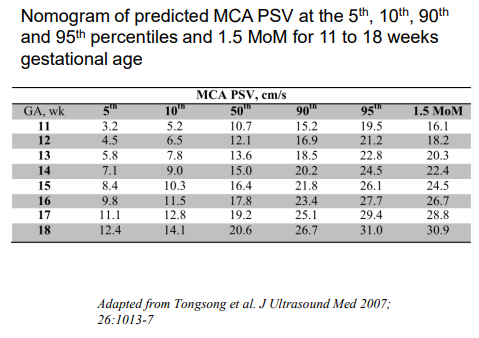
Overall, there is a 12% false positive rate, which is more frequent after former fetal transfusions. Some more recent studies have also looked at cardiac function assessment with 73% of providers in the international expert Delphi consensus on monitoring and management of hemolytic disease reporting utilization of cardiac size and valvular regurgitation as parameters to assess fetal anemia, though not to guide management such as with intrauterine transfusion. In red cell isoimmunized pregnancies, the left and right cardiac output is increased in proportion to the degree of fetal anemia.
MCA Dopplers (see Figure 2) are usually initiated at 16 weeks of gestation (though in some cases may be performed as early as 15 weeks). If there is a true fetal anemia suspected after MCA Doppler surveillance, the next step is to proceed with percutaneous umbilical vein sampling (PUBS) and intrauterine transfusion (IUT). During this procedure, fetal blood is sampled through the umbilical vein and hemoconcentrated, leukoreduced, irradiated CMV negative blood is transfused into the fetus to bring the hematocrit (HCT) to at least 45 (40-50 g/dL). Generally, a goal hematocrit of 75-80% should be achieved in the blood sample for transfusion in order to reduce the volume of blood needed to infuse into the fetus, and decrease risk of fetal compromise from volume overload and increased strain on the fetal heart.
Figure 2
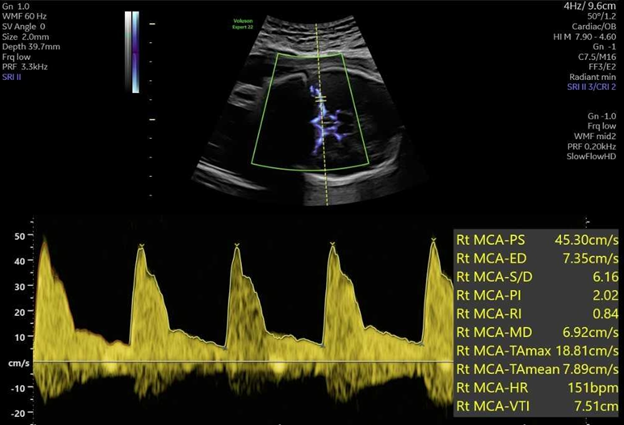
Umbilical vein access is best obtained at the placental cord insertion, followed by a free floating loop of cord or the intrahepatic vein alternatively. Umbilical vein insertion into the abdominal wall is generally avoided due to concern for possible fetal bradycardia. Generally PUBS is reserved until after 20-22 weeks gestation due to higher risk of fetal complications at earlier gestations, however, intrahepatic and intraperitoneal transfusions can be performed even as early as 17 - 18 weeks in special cases.
Some centers may follow MCA-PSV after the first one or two transfusions to guide subsequent transfusion timing, though MCA Dopplers may be less reliable after multiple transfusions of adult red blood cells. They may also be less reliable later in the third trimester. More commonly, there is a recommended interval by which further transfusions can be guided based on anticipated decline in fetal hemoglobin over time (generally thought to be about 1g/dL every three days). Some centers will use empiric intervals to time transfusions.
In patients who have negative paternal antigen testing or where a critical antibody titer is never reached, there is no change to the delivery plan.
If significant anemia develops > 35 weeks, delivery with postnatal management of hemolytic anemia is generally recommended versus undergoing the risk of an intrauterine procedure.
Patients with mild anemia who never required IUT can be delivered early term, at 37 - 38 weeks gestation. Generally patients who have undergone IUTs will be delivered between two to three weeks after the last PUBS procedure (usually not completed past 34-35 weeks gestation).
In general, the first child affected by alloimmunization will develop a less severe course - usually a milder anemia that presents later in gestation. If there has been a prior affected pregnancy, it can present with a more severe fetal anemia at an earlier gestation—a 2018 study showed average development of anemia nine days earlier in subsequent pregnancies as compared to previous pregnancies.
In the case where a patient has previously had an affected fetus or newborn, surveillance is often started with Dopplers versus awaiting a critical titer. A baseline maternal antibody is often obtained as well to help guide intervention in early pregnancy (e.g. for immunomodulation vs. waiting for availability of fetal intervention in the second trimester).
In some cases, patients can elect to undergo IVF to select for unaffected embryos if the father is heterozygous for a red cell antigen that has the possibility to cause hydrops fetalis. Use of donor sperm or a gestational carrier are generally less common but alternately available options. There are also new medications in development to help reduce risks to subsequent pregnancies, and these are further described below.
Fetal hydrops can be due to multiple other causes aside from alloimmunization - this is known as non-immune hydrops including underlying heart defects and fetal arrhythmias or abnormal heart rhythms, infections, genetic syndromes, other blood cell disorders (such as thalassemia, sickle cell disease, and hereditary spherocytosis), and fetal tumors. Placental abnormalities and tumors can also lead to fetal anemia and hydrops. Twins can be at risk of fetal hydrops through the process of twin-twin transfusion syndrome. Currently we are seeing an increased incidence of anemia due to Parvovirus. Parvovirus is a small single stranded DNA virus sometimes known as fifth disease that presents with mild rash and fevers in children and joint pain in adults, but that can lead to fetal hemolytic anemia by destruction of fetal red blood cells in a similar process seen with alloimmunization. This can be identified by obtaining a thorough clinical history and maternal serum screening.
Currently therapeutic management in most centers is limited to percutaneous umbilical blood sampling (PUBS) and blood transfusion, intravenous immunoglobulin (IVIG) and plasmapheresis. There is a new study ongoing through Johnson & Johnson that is looking at use of monoclonal antibodies. Monoclonal antibodies can be used to inhibit the maternal immune response and potentially delay or prevent fetal anemia before invasive intervention such as PUBS is necessary (see below for clinicians on more details of the AZALEA Phase 3 trial).
Patients should be referred to the Fetal Care Center with the diagnosis of elevated MCA Dopplers. If Dopplers are > 1.5 MoM, we recommend not utilizing Betamethasone until after evaluation with a Fetal Care Center as this can falsely lower MCA Dopplers in the immediate time period. If a patient has a previous pregnancy affected by alloimmunization (especially hydrops fetalis of the fetus or newborn), the patient can be referred in the preconception time period to discuss care and management of future pregnancies.
This can be considered in patients with a history of very high titers or of prior fetal loss secondary to early hydrops or early transfusion (known as early onset hemolytic disease of the fetus and newborn or EOS - HDFN). Currently immunomodulation is performed through plasmapheresis and IVIG (Ruma et al., Zwiers et al.). This is based on the PETIT study which showed that initiating IVIG prior to 13 weeks in patients with a history of severe fetal anemia, can postpone anemia onset by 25 days which significantly improves the ability to treat. There is new research looking into delaying or preventing severe fetal anemia through the use of Nipocalimab - a monoclonal antibody. The phase 2 Unity study has concluded and found that 54% of participants achieved a live birth after 32 weeks without needing intrauterine transfusion. They are now enrolling for their Phase 3 AZALEA clinical study.
- Anabusi S, Van Mieghem T, Ryan G, Shinar S. Elevated Middle Cerebral Artery Peak Systolic Velocity in Non-Anemic Fetuses: Providing a Better Understanding of Enigmatic Middle Cerebral Artery Peak Systolic Velocity. Fetal Diagn Ther. 2024;51(6):550-558.
- Jacobs JW, Booth GS, Raza S, Clark LM, Fasano RM, Gavriilaki E, Abels EA, Binns TC, Duque MA, McQuilten ZK, Mingot-Castellano ME, Savani BN, Sharma D, Tran MH, Tormey CA, Moise KJ Jr, Bloch EM, Adkins BD. Current state and potential applications of neonatal Fc receptor (FcRn) inhibitors in hematologic conditions. Am J Hematol. 2024 Dec;99(12):2351-2366.
- Maisonneuve E, Jayot A, Friszer S, Castaigne V, Cynober E, Pernot F, Mailloux A, Jouannic JM, Cortey A, Carbonne B. Accuracy of Middle Cerebral Artery Doppler Assessment between 34 and 37 Weeks in Fetuses with Red Cell Alloimmunization. Fetal Diagn Ther. 2017;42(3):225-231. doi: 10.1159/000456661. Epub 2017 Mar 10. PMID: 28278506.
- Management and prevention of red cell alloimmunization in pregnancy: a systematic review (Level I) Obstet Gynecol. 2012; 120:1132-1139
- Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ Jr, Dorman KF, Ludomirsky A, Gonzalez R, Gomez R, Oz U, Detti L, Copel JA, Bahado
- Singh R, Berry S, Martinez-Poyer J, Blackwell SC. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med 2000; 342: 9–14
- Ronan P. Sugrue,Kenneth J. Moise,Jerome J. Federspiel,Elizabeth Abels,Judy Z. Louie,Zhen Chen,Lance Bare,Damian P. Alagia,Harvey W. Kaufman, Maternal red blood cell alloimmunization prevalence in the United States, Blood Adv, 2024
- Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #8: The fetus at risk for anemia–diagnosis and management. Mari, Giancarlo et al. American Journal of Obstetrics & Gynecology, Volume 212, Issue 6, 697 - 710
- Variations in antenatal management and outcomes in haemolytic disease of the fetus and newborn: an international, retrospective, observational cohort study de Winter, Derek P et al.The Lancet Haematology, Volume 11, Issue 12, e927 - e937
- Zwiers C, et al. . Postponing Early intrauterine Transfusion with Intravenous immunoglobulin Treatment; the PETIT study on severe hemolytic disease of the fetus and newborn. Am J Obstet Gynecol. 2018 Sep;219(3):291.e1-291.e9.
- Crombleholme TM, Lim F, Peiro JL, Rintoul NE, Simpson LL. eds. Fetal and Neonatal Surgery and Medicine. McGraw Hill; 2024.